UC
EFFICACY
RESULTS
INDICATION
RINVOQ is indicated for the treatment of adults with moderately to severely active ulcerative colitis (UC) who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers. If TNF blockers are clinically inadvisable, patients should have received at least one approved systemic therapy prior to use of RINVOQ.
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biological therapies for UC, or with potent immunosuppressants such as azathioprine and cyclosporine.
Durable Remission1,2
Clinical remission* at Week 8 and Week 52
Remission data observed at Week 100†
†Based on not statistically significant data. No conclusions can be made.
Powerful Healing1
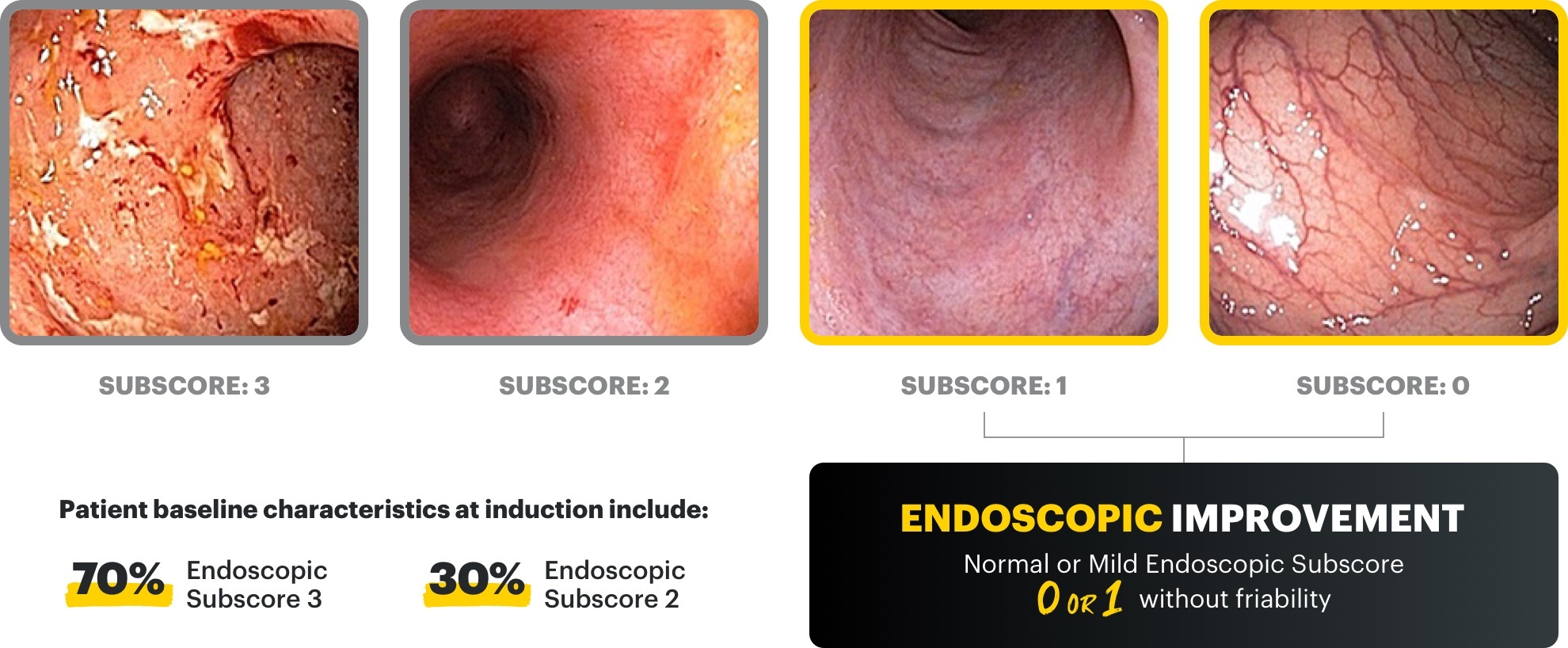
Endoscopic improvement‡ and histo‑endoscopic mucosal improvement§ at Week 8 and Week 52
#The relationship between histo-endoscopic mucosal improvement to disease progression and long-term outcomes was not evaluated.
Rapid Relief1
Rapid relief of rectal bleeding and stool frequency evaluated at Week 2¶
*Clinical remission per modified Mayo Score is defined as stool frequency subscore ≤1 and not greater than baseline, rectal bleeding subscore of 0, and endoscopic subscore ≤1 without friability.
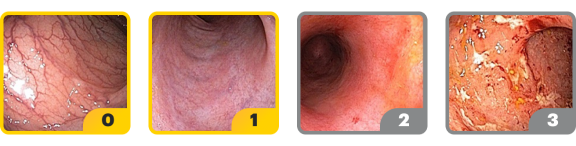
‡Endoscopic improvement was defined as Mayo endoscopic subscore of 0 or 1 without friability. Endoscopic results are based on a full colonoscopy or flexible sigmoidoscopy, depending on the extent of disease at study entry.
§Histo-endoscopic mucosal improvement was defined as Mayo endoscopy subscore of 0 or 1 without friability and Geboes score ≤3.1 (neutrophil infiltration in <5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue). Endoscopic results are based on a full colonoscopy of flexible sigmoidoscopy, depending on the extent of disease at study entry, and histology results are based on a set of 2 biopsies.
¶Clinical response per partial modified Mayo Score is a composite of Mayo stool frequency and rectal bleeding subscores and is defined as a decrease in total score ≥30% and ≥1 point from baseline and a decrease in rectal bleeding subscore ≥1 or rectal bleeding subscore of 0 or 1.
IR=intolerance or inadequate response; TNFi=tumor necrosis factor inhibitor.
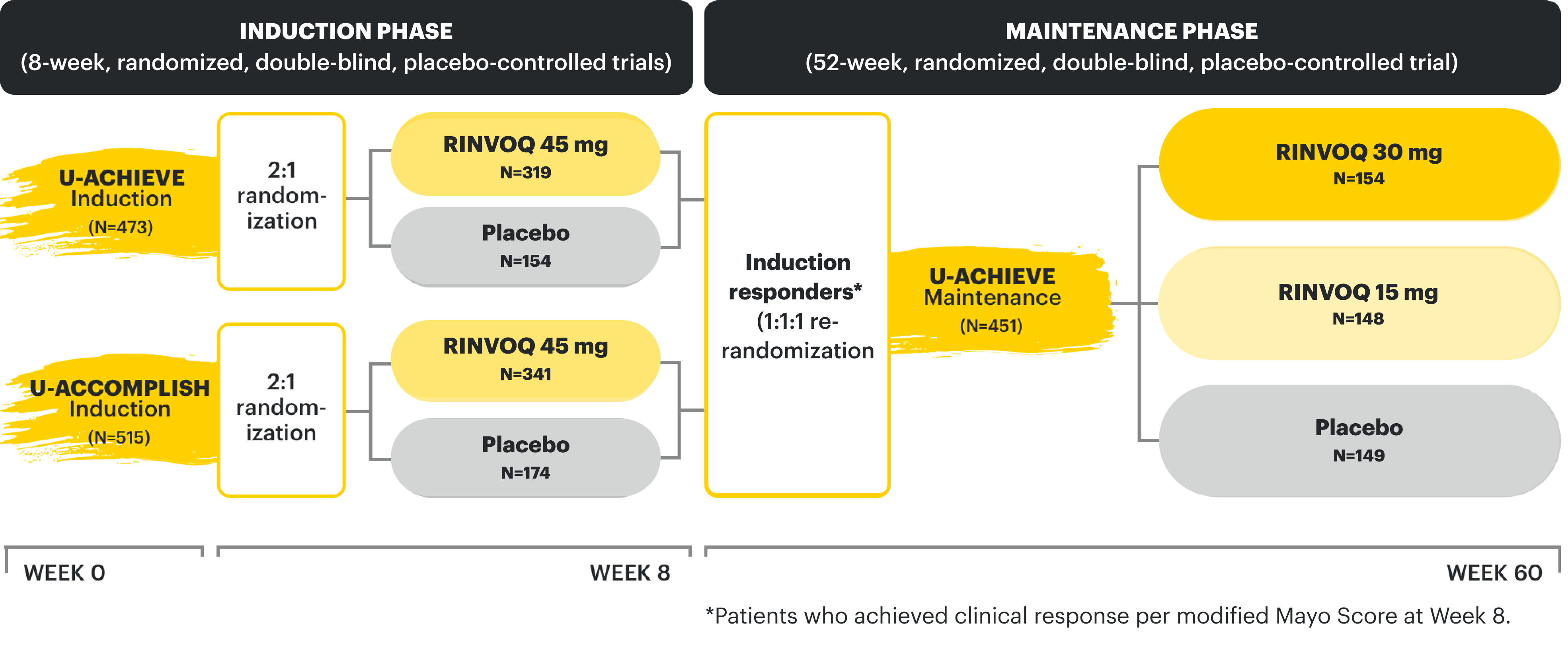
U-ACHIEVE Induction and U-ACCOMPLISH Induction Study Design Intro1: 8-week, double-blind, placebo-controlled, Phase 3 clinical studies of 988 patients (473 patients for U-ACHIEVE and 515 patients for U-ACCOMPLISH) with moderately to severely active UC and demonstrated prior treatment failure to oral aminosalicylates, corticosteroids, immunosuppressants, and/or biologic treatment. Patients were randomized to receive either RINVOQ 45 mg or placebo once daily for 8 weeks. The primary endpoint was clinical remission per modified Mayo Score at Week 8.
U-ACHIEVE Maintenance Study Design Intro1: 52-week, double-blind, placebo-controlled, Phase 3 clinical study of 746 patients who achieved clinical response per modified Mayo Score (a decrease in total score ≥30% and ≥2 points from baseline and a decrease in rectal bleeding subscore ≥1 or rectal bleeding subscore of 0 or 1) during induction with 8-week RINVOQ 45 mg once daily and were re-randomized to the maintenance study. The primary efficacy analysis population was the first randomized 451 patients. Patients were randomized to receive RINVOQ 15 mg, 30 mg, or placebo once daily for up to 52 weeks. The primary endpoint was clinical remission per modified Mayo Score at Week 52.
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
Durable Remission
at Week 8 and 52
Clinical Remission
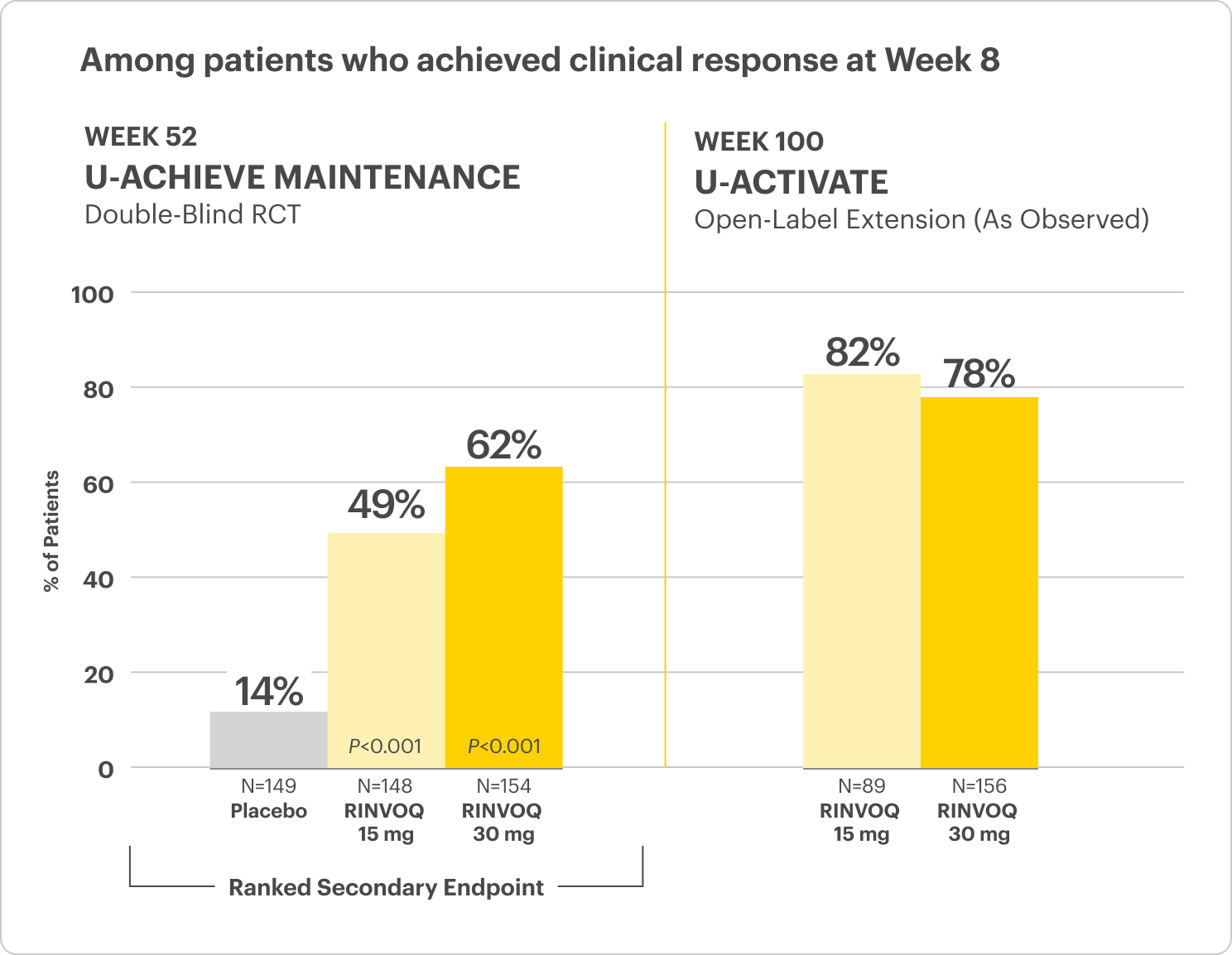
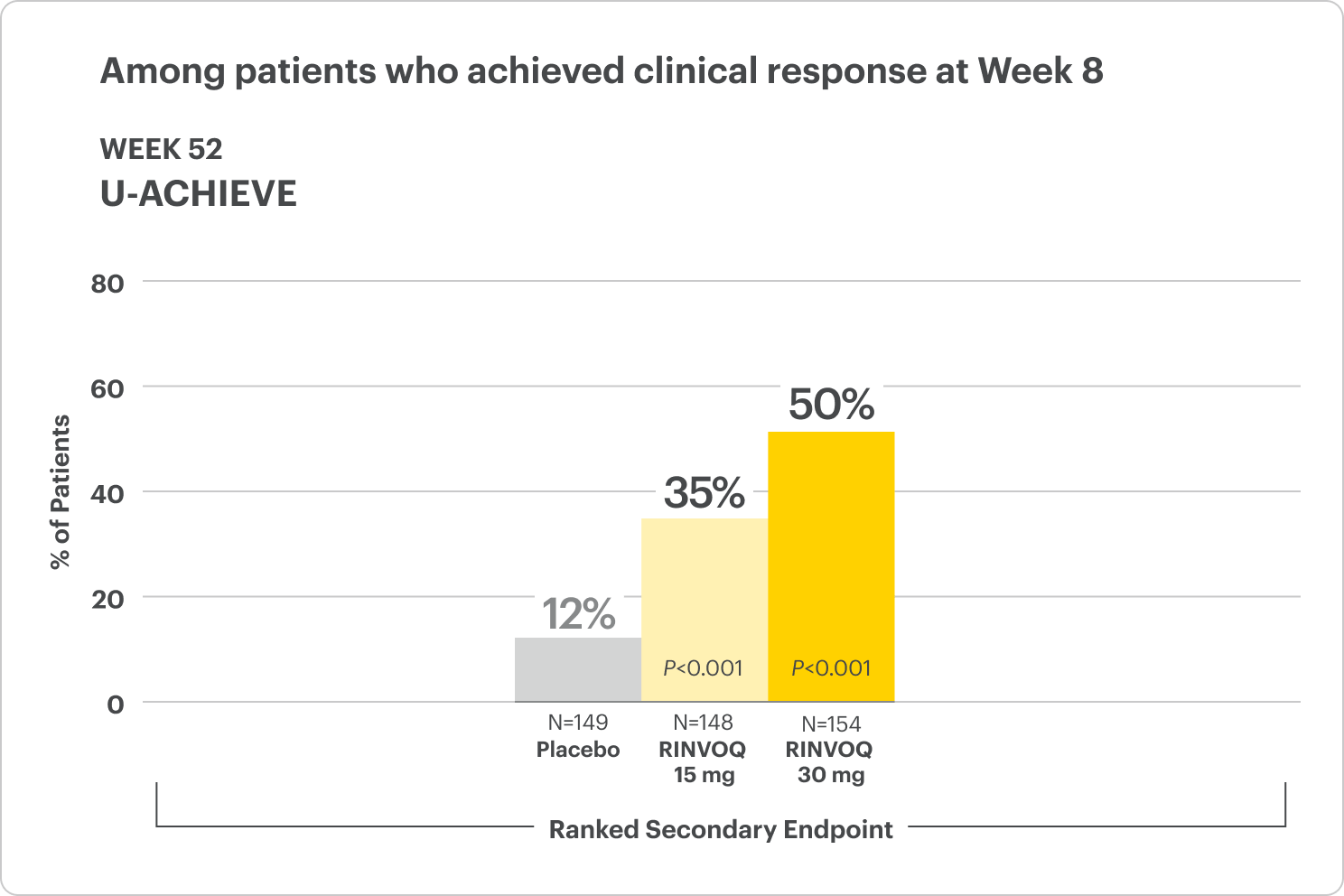
Clinical Remission at Week 52 and Up to ~2 Years1,2
Among patients who achieved clinical response at Week 8
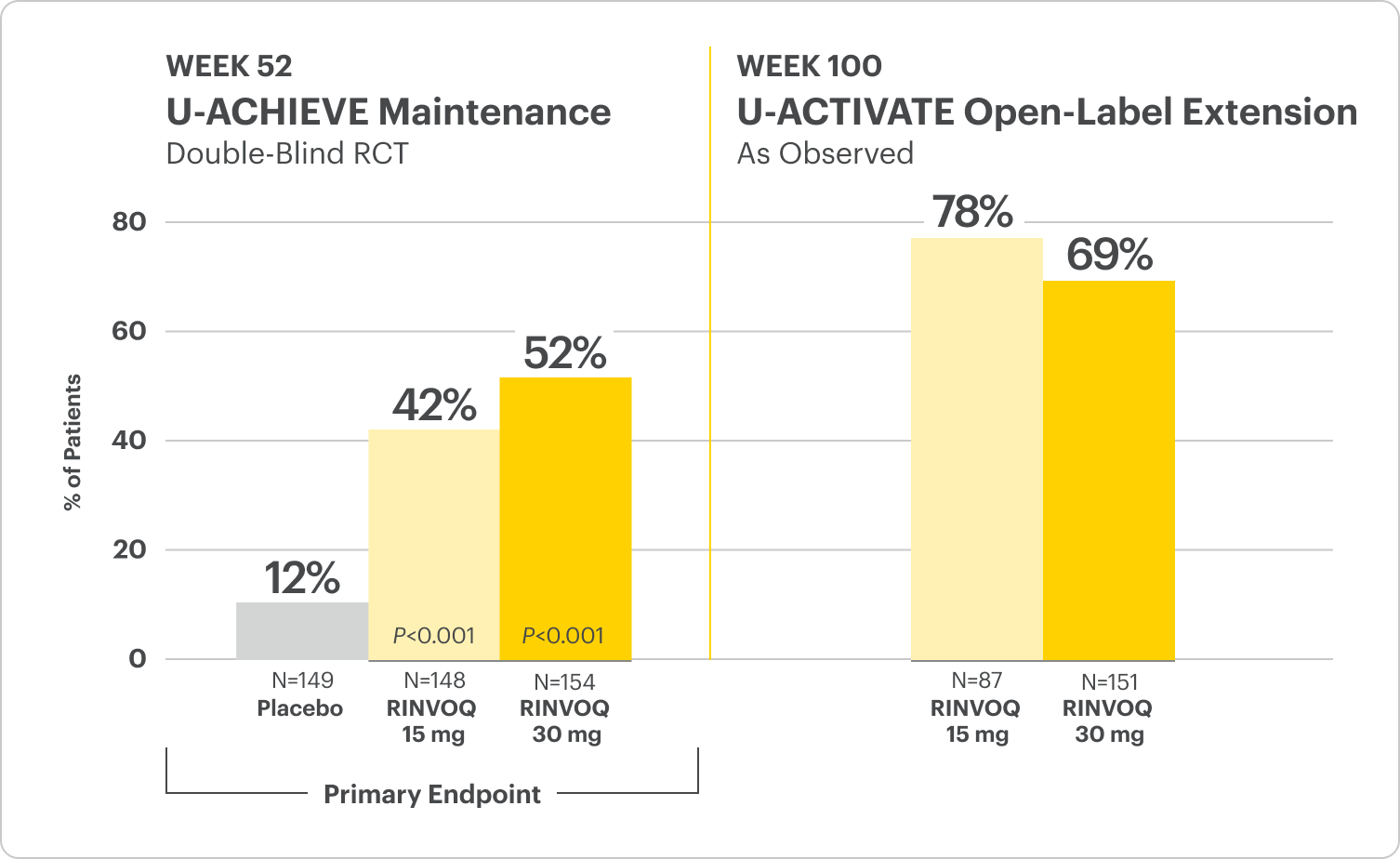
All P-values are RINVOQ treatment arms vs placebo.
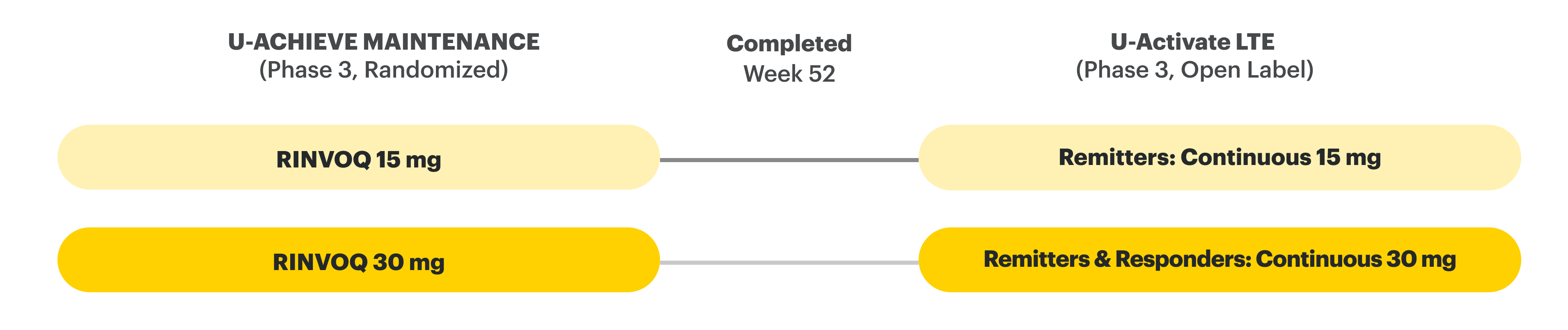
In the LTE analysis, the data are segmented as follows:
- RINVOQ 15 mg arm: Patients who achieved clinical remission (remitters) on RINVOQ 15 mg at Week 52 of maintenance and received continuous RINVOQ 15 mg in the LTE period (N=101)
- RINVOQ 30 mg arm: All patients on RINVOQ 30 mg who completed Week 52 of maintenance (remitters and patients in clinical response) and received continuous RINVOQ 30 mg in the LTE period (N=175)
OLE LIMITATIONS: In an OLE, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out.
AO DISCLOSURE: In an as-observed (AO) analysis, missing visit data were excluded from calculations for that visit, which may increase the percent of responders. All observed data were used regardless of premature discontinuation of study drug, or initiation of concomitant medications. The same patient may not have a response at each timepoint.
RECOMMENDED MAINTENANCE DOSING: A dose of 30 mg once daily may be considered in patients with refractory, severe, or extensive disease. Discontinue if therapeutic response is not achieved with the 30 mg dose.
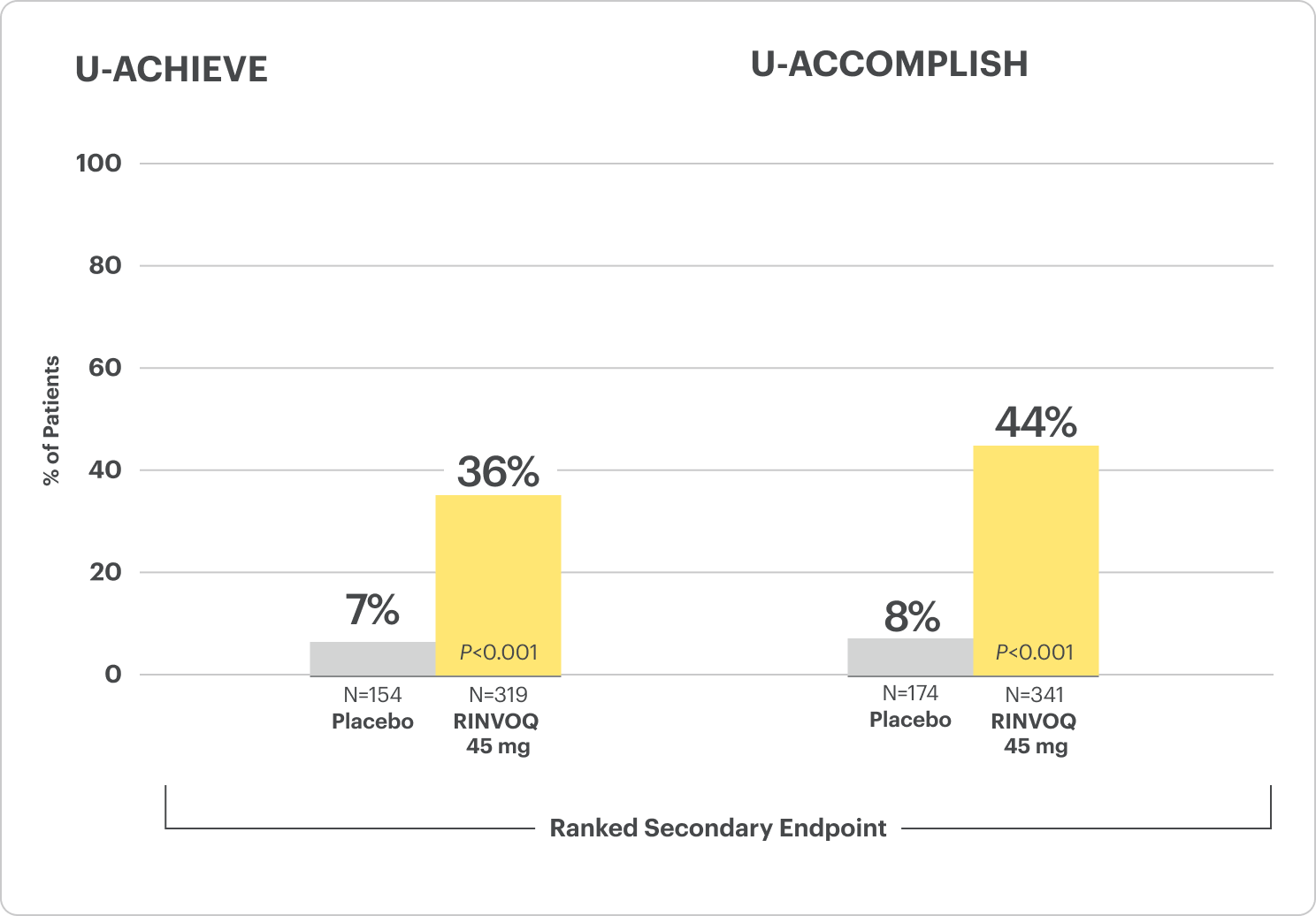
Corticosteroid-Free Clinical Remission at Week 521
Among patients who achieved remission at Week 8
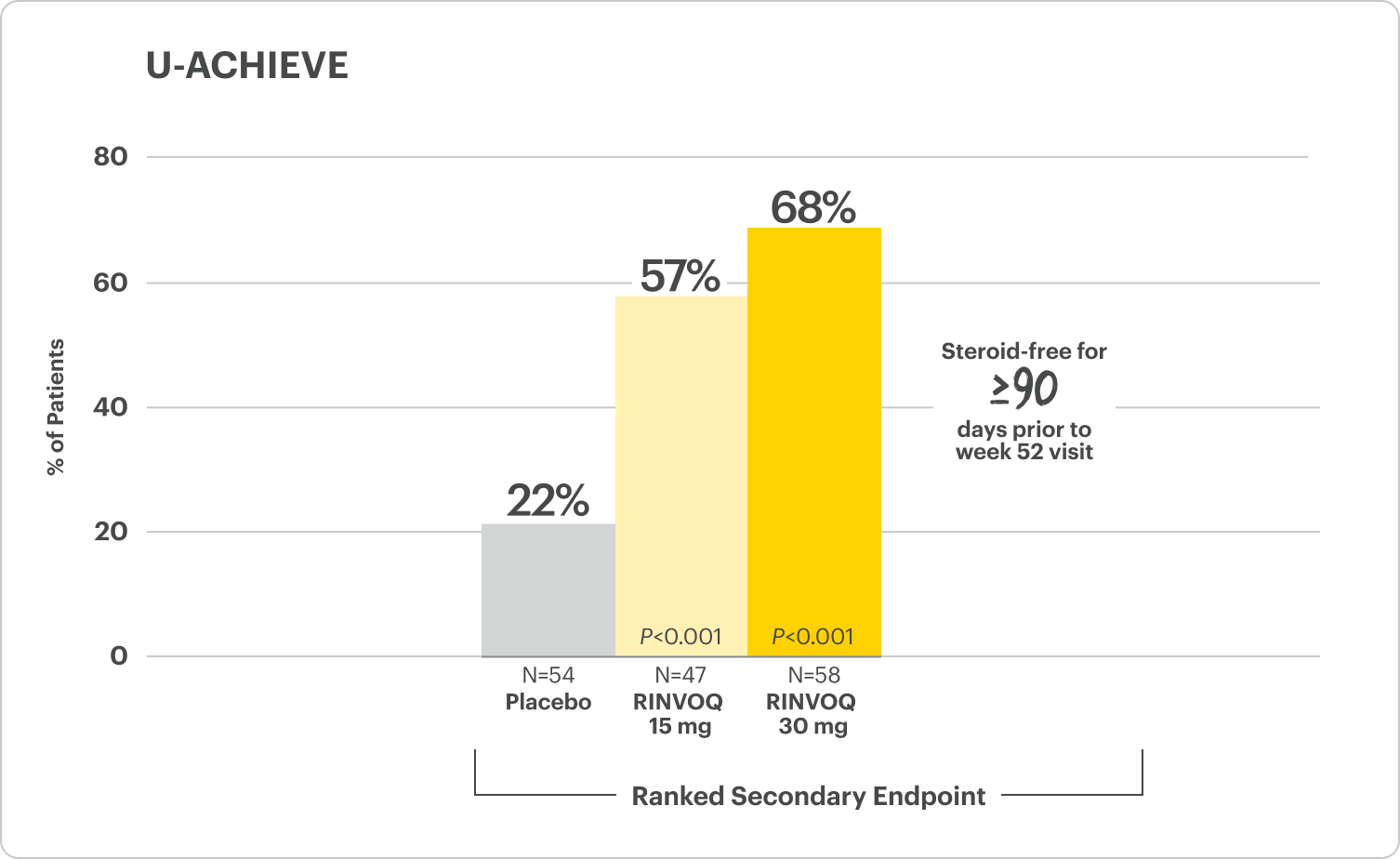
All P-values are RINVOQ treatment arms vs placebo.
RECOMMENDED MAINTENANCE DOSING: A dose of 30 mg may be considered in patients with refractory, severe, or extensive disease. Discontinue if therapeutic response is not achieved with the 30 mg dose.
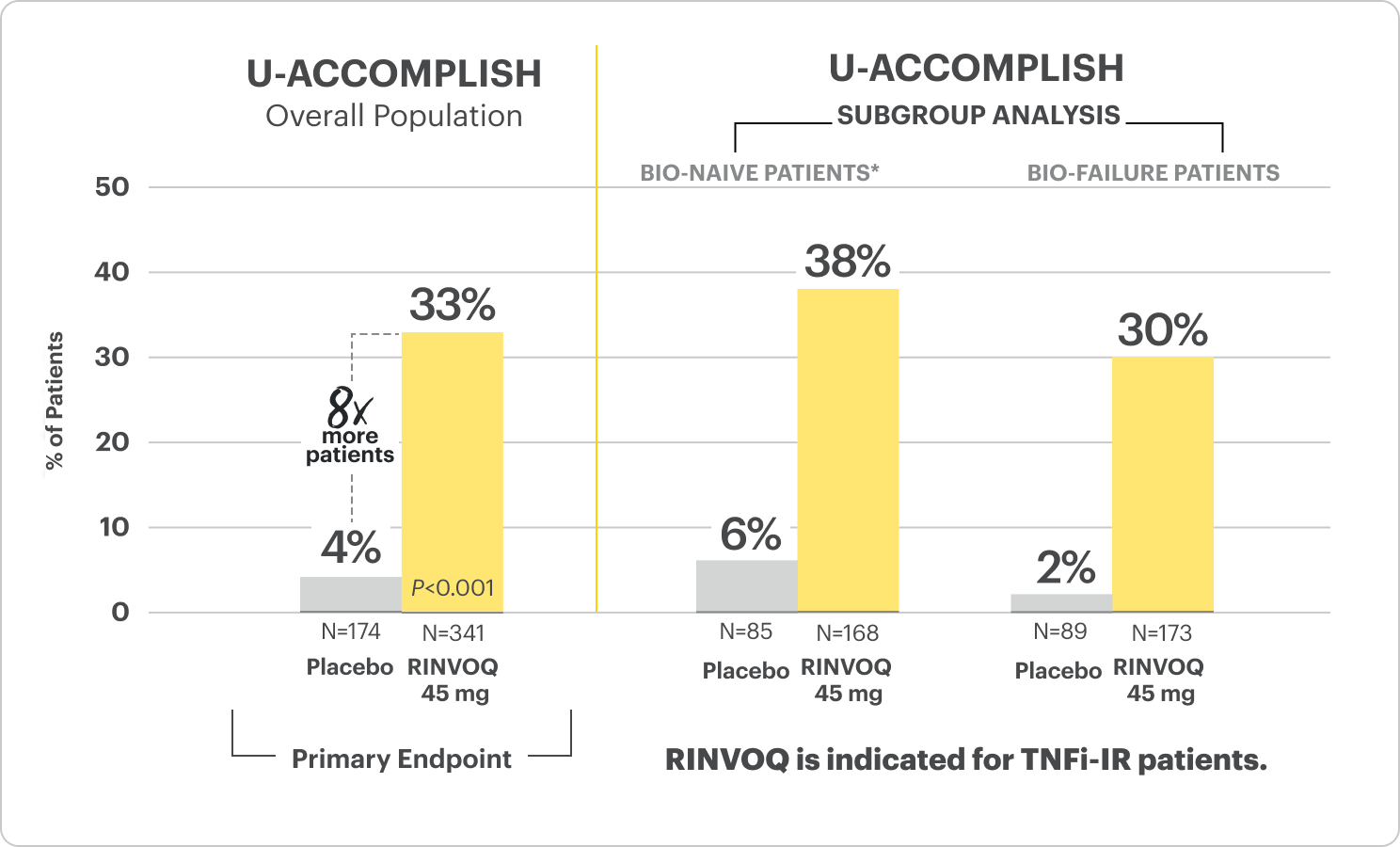
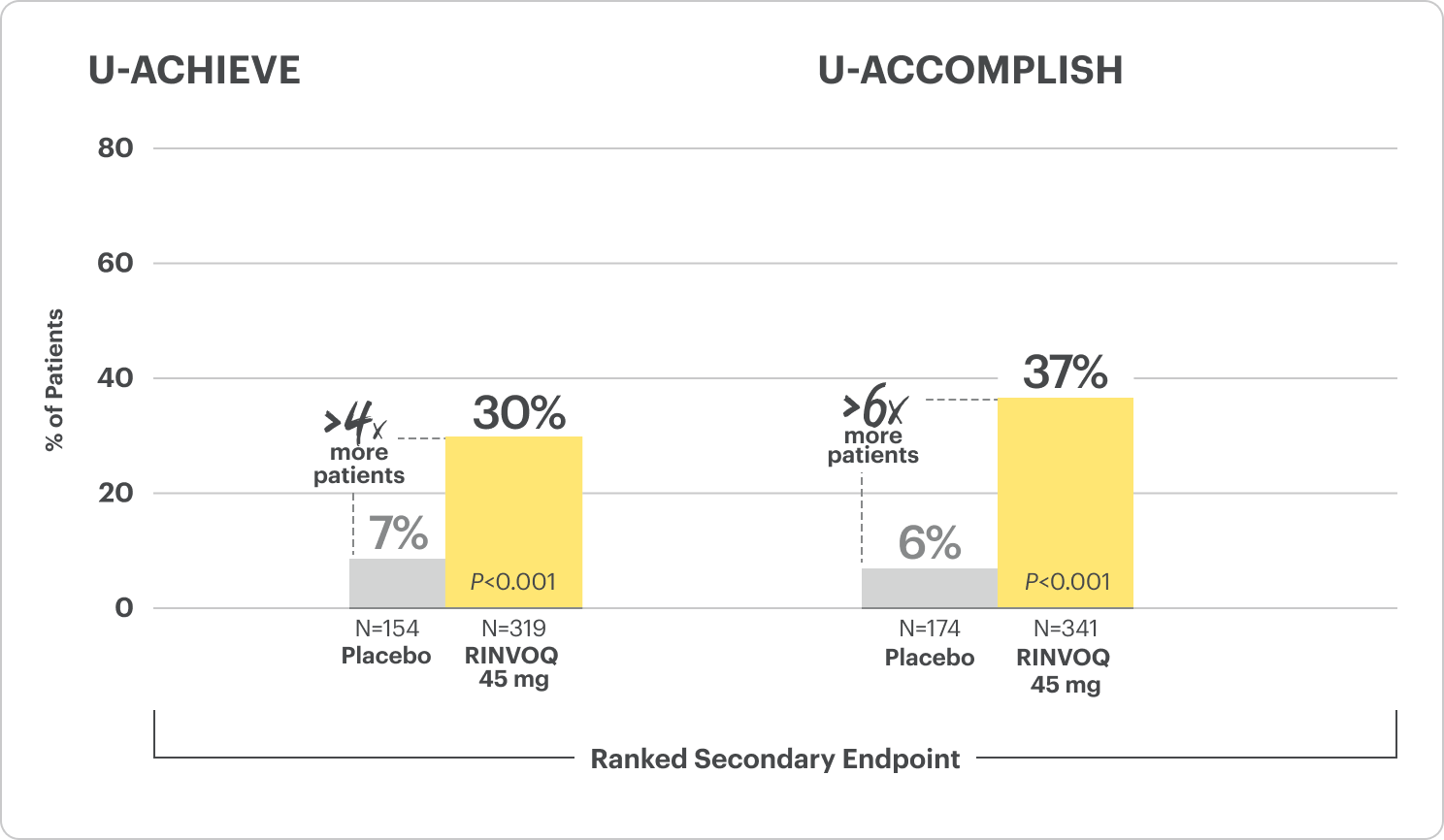
Clinical Remission at Week 81
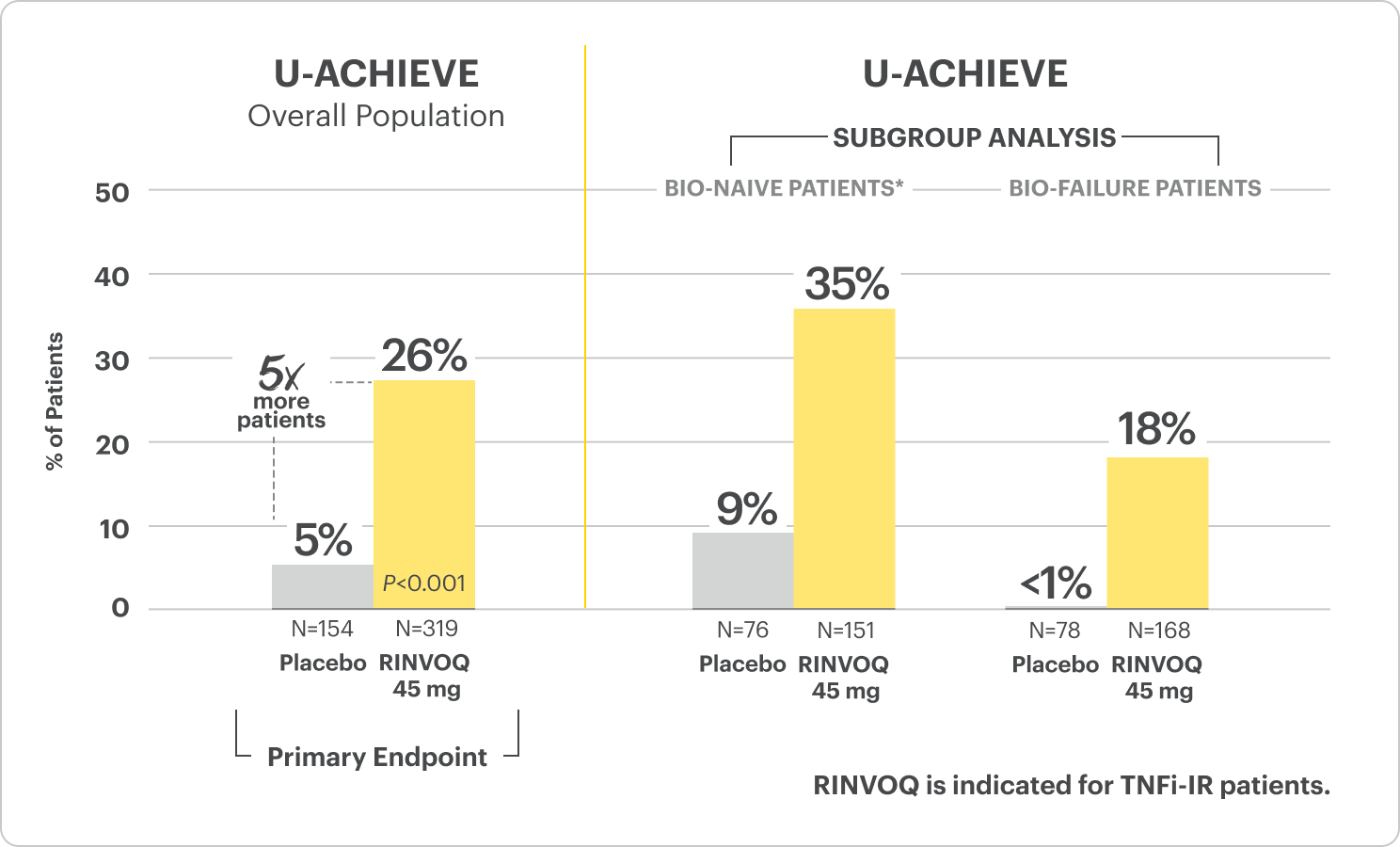
*Bio-naive patients include patients who were exposed to a biologic but did not experience treatment failure.
All P-values are RINVOQ treatment arms vs placebo.
RECOMMENDED MAINTENANCE DOSING
A dose of 30 mg may be considered in patients with refractory, severe, or extensive disease. Discontinue if therapeutic response is not achieved with the 30 mg dose.
*Bio-naive patients include patients who were exposed to a biologic but did not experience treatment failure.
All P-values are RINVOQ treatment arms vs placebo.
RECOMMENDED MAINTENANCE DOSING
A dose of 30 mg may be considered in patients with refractory, severe, or extensive disease. Discontinue if therapeutic response is not achieved with the 30 mg dose.
Powerful Healing
at Weeks 8 and 52
Endoscopic Improvement & Histo-endoscopic Mucosal Improvement
Endoscopic Improvement1,2 (Endoscopy subscore of 0 or 1, without friability)
All P-values are RINVOQ treatment arms vs placebo.
RECOMMENDED MAINTENANCE DOSING: A dose of 30 mg may be considered in patients with refractory, severe, or extensive disease. Discontinue if therapeutic response is not achieved with the 30 mg dose.
All P-values are RINVOQ treatment arms vs placebo.
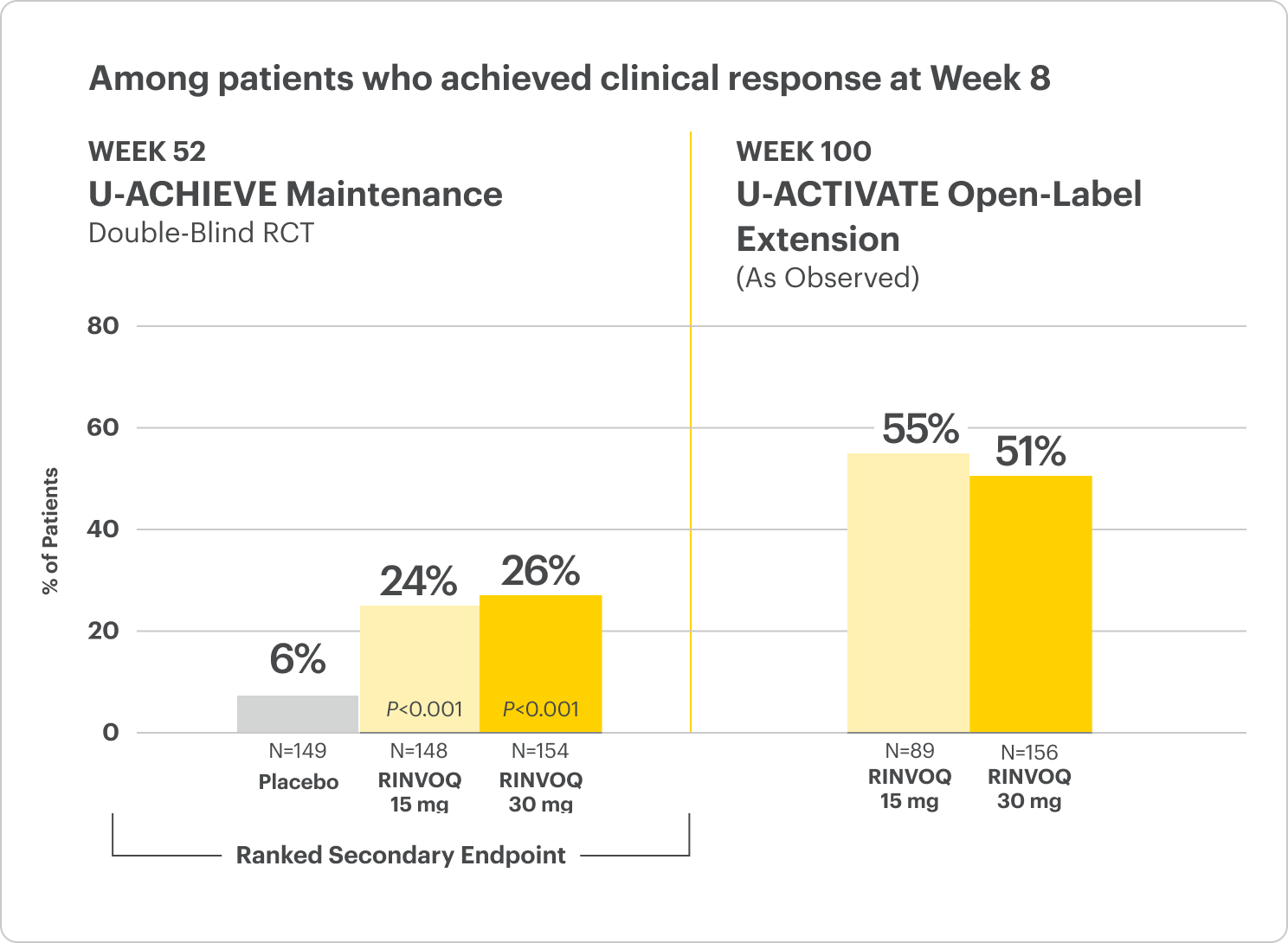
OLE LIMITATIONS: In an OLE, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out.
AO DISCLOSURE: In an-observed (AO) analysis, missing visit data were excluded from calculations for that visit, which may increase the percent of responders. All observed data were used regardless of premature discontinuation of study drug, or initiation of concomitant medications. The same patient may not have a response at each timepoint.
RECOMMENDED MAINTENANCE DOSING
A dose of 30 mg may be considered in patients with refractory, severe, or extensive disease. Discontinue if therapeutic response is not achieved with the 30 mg dose.
In the LTE analysis, the data are segmented into 3 groups:
- RINVOQ 15 mg arm: Patients who achieved clinical remission (remitters) on RINVOQ 15 mg at Week 52 of Maintenance Trial and received continuous RINVOQ 15 mg in LTE period (n=101)
- RINVOQ 30 mg arm: All patients on RINVOQ 30 mg who completed Week 52 of Maintenance Trial (remitters and responders) and received continuous RINVOQ 30 mg in the LTE period (n=175)
Endoscopic Remission1,2 (Endoscopy subscore of 0)
All P-values are RINVOQ treatment arms vs placebo.
OLE LIMITATIONS: In an OLE, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out.
AO DISCLOSURE: In an-observed (AO) analysis, missing visit data were excluded from calculations for that visit, which may increase the percent of responders. All observed data were used regardless of premature discontinuation of study drug, or initiation of concomitant medications. The same patient may not have a response at each timepoint.
RECOMMENDED MAINTENANCE DOSING
A dose of 30 mg may be considered in patients with refractory, severe, or extensive disease. Discontinue if therapeutic response is not achieved with the 30 mg dose.
Histo-endoscopic Mucosal Improvement1 (Endoscopic subscore of 0 or 1, without friability and histologic improvement
with Geboes score ≤3.1)
All P-values are RINVOQ treatment arms vs placebo.
The relationship between this endpoint to disease progression and long-term outcomes was not evaluated.
RECOMMENDED MAINTENANCE DOSING
A dose of 30 mg may be considered in patients with refractory, severe, or extensive disease. Discontinue if therapeutic response is not achieved with the 30 mg dose.
All P-values are RINVOQ treatment arms vs placebo.
The relationship between this endpoint to disease progression and long-term outcomes was not evaluated.
RECOMMENDED MAINTENANCE DOSING
A dose of 30 mg may be considered in patients with refractory, severe, or extensive disease. Discontinue if therapeutic response is not achieved with the 30 mg dose.
Safety Considerations
Serious Infections: RINVOQ-treated patients are at increased risk of serious bacterial (including tuberculosis [TB]), fungal, viral, and opportunistic infections leading to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids.
Mortality: A higher rate of all-cause mortality, including sudden cardiovascular (CV) death, was observed with a Janus kinase inhibitor (JAKi) in a study comparing another JAKi with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients ≥50 years with ≥1 CV risk factor.
Malignancies: Malignancies have occurred in RINVOQ-treated patients. A higher rate of lymphomas and lung cancer (in current or past smokers) was observed with another JAKi when compared with TNF blockers in RA patients.
Major Adverse Cardiovascular Events: A higher rate of CV death, myocardial infarction, and stroke was observed with a JAKi in a study comparing another JAKi with TNF blockers in RA patients ≥50 years with ≥1 CV risk factor. History of smoking increases risk.
Thromboses: Deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated for inflammatory conditions with JAK inhibitors, including RINVOQ. A higher rate of thrombosis was observed with another JAKi when compared with TNF blockers in RA patients.
Hypersensitivity: RINVOQ is contraindicated in patients with hypersensitivity to RINVOQ or its excipients.
Other Serious Adverse Reactions: Hypersensitivity Reactions, Gastrointestinal Perforations, Laboratory Abnormalities, and Embryo-Fetal Toxicity.
Rapid Relief
of Rectal Bleeding and Stool Frequency at Week 2
Clinical Response at Weeks 2, 4, 6, and 84
Composite of Rectal Bleeding and Stool Frequency Subscores
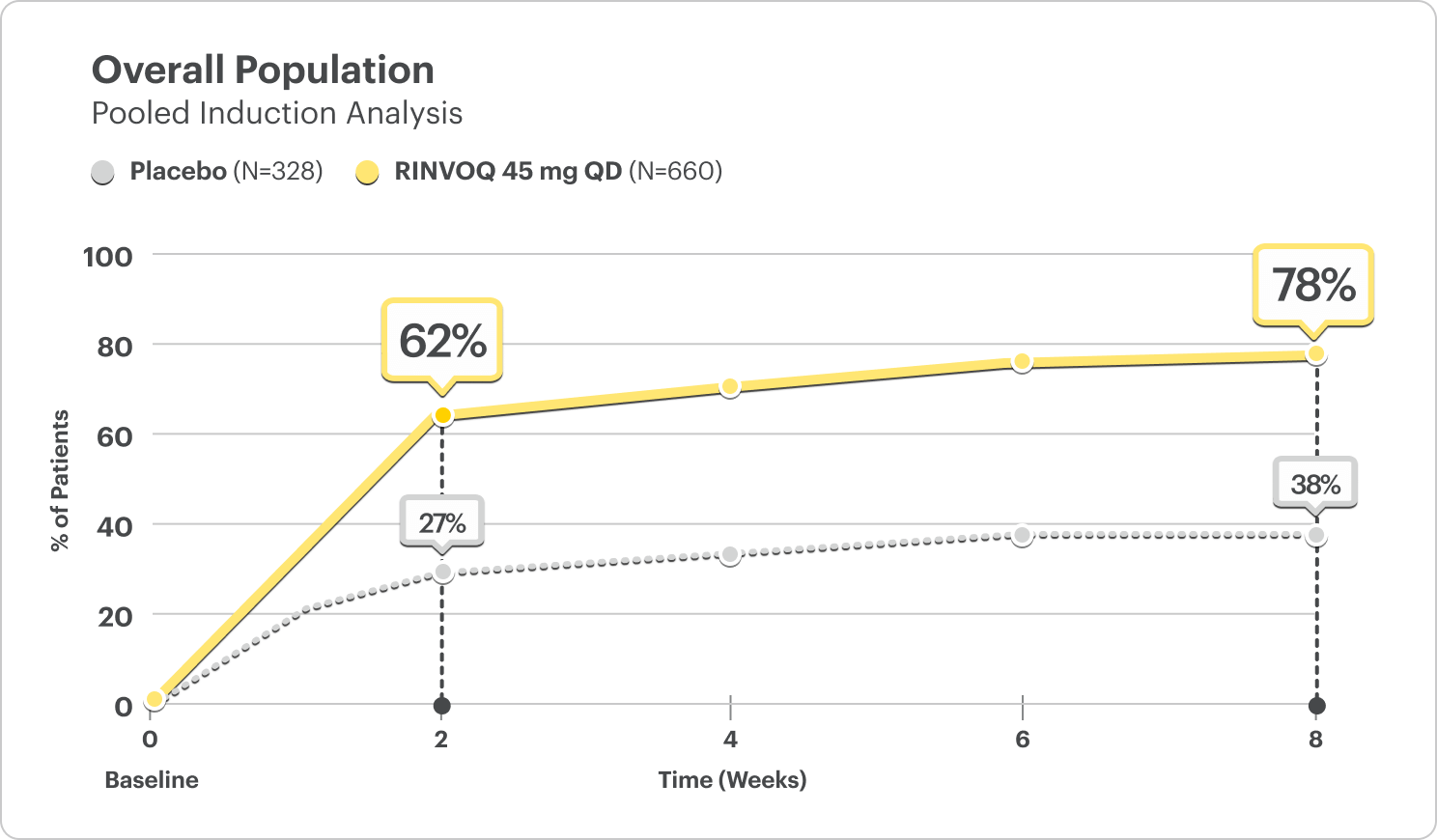
CLINICAL RESPONSE AT WEEK 2:
- U-ACHIEVE Induction: 60% on RINVOQ 45 mg vs 27% on placebo (P<0.001)3
- U-ACCOMPLISH Induction: 63% on RINVOQ 45 mg vs 26% on placebo (P<0.001)3
DATA LIMITATIONS: The pre-specified integrated analysis for Clinical Response at Week 2 and over time is considered supportive of the efficacy results obtained from the individual studies and is intended to be interpreted within this context. No multiplicity adjustment was performed; thus, no statistical inferences can be made.
Rectal Bleeding Subscore (RBS) Data by Day5
(Daily Patient Diary Data)
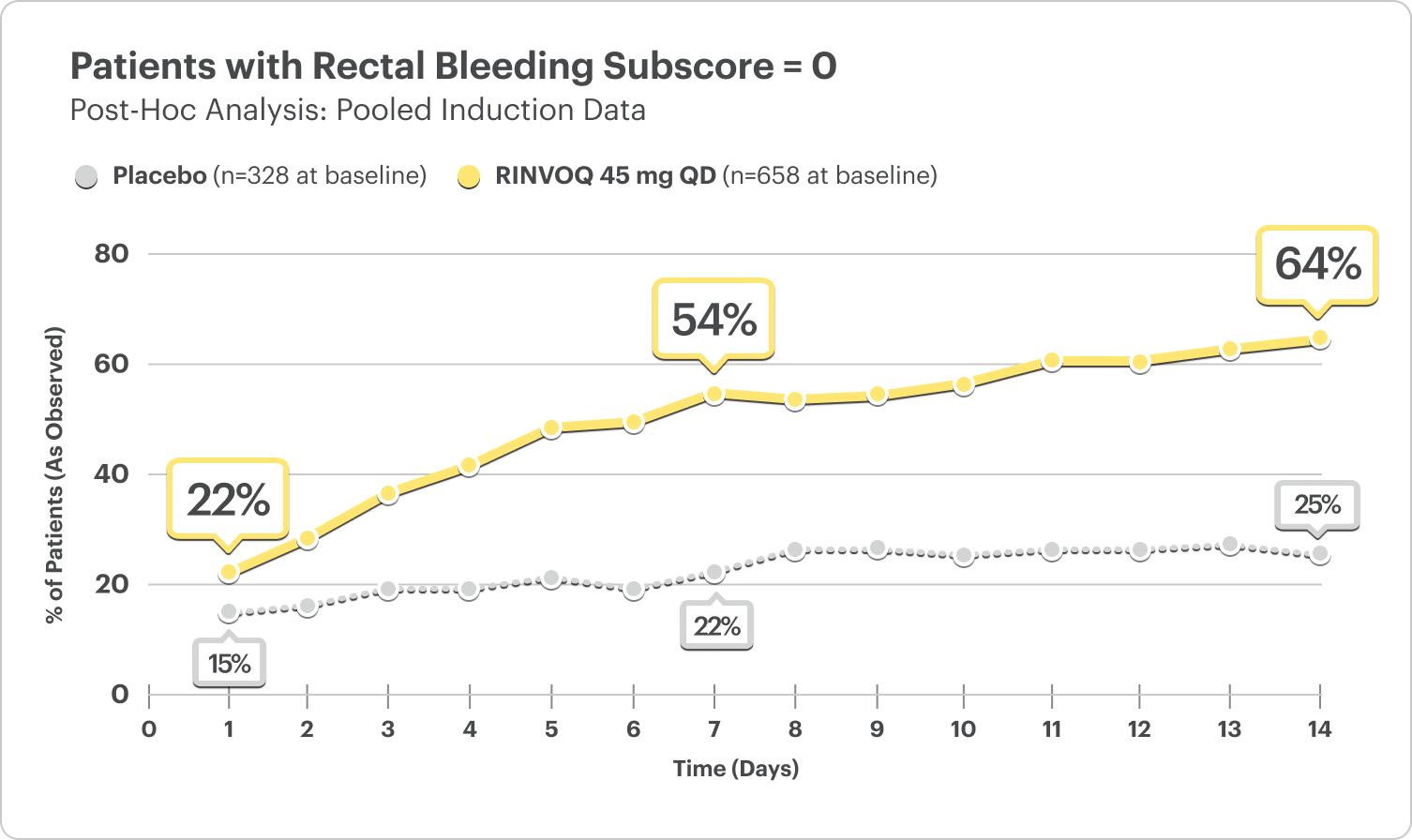
- At Day 1, 22% of patients on RINVOQ 45 mg reported no rectal bleeding vs 15% on placebo5
- At Week 1, 54% of patients on RINVOQ 45 mg reported no rectal bleeding vs 22% on placebo5
DATA LIMITATIONS: Post hoc analyses were not adjusted for multiplicity; thus, no statistical inferences can be made. These analyses utilized an as-observed approach and did not impute values for missing evaluations.
Stool Frequency Subscore (SFS) Data by Day5
(Daily Patient Diary Data)
Patients with a reduction in stool frequency subscore ≤1 point
Post Hoc Analysis: Pooled Induction Data
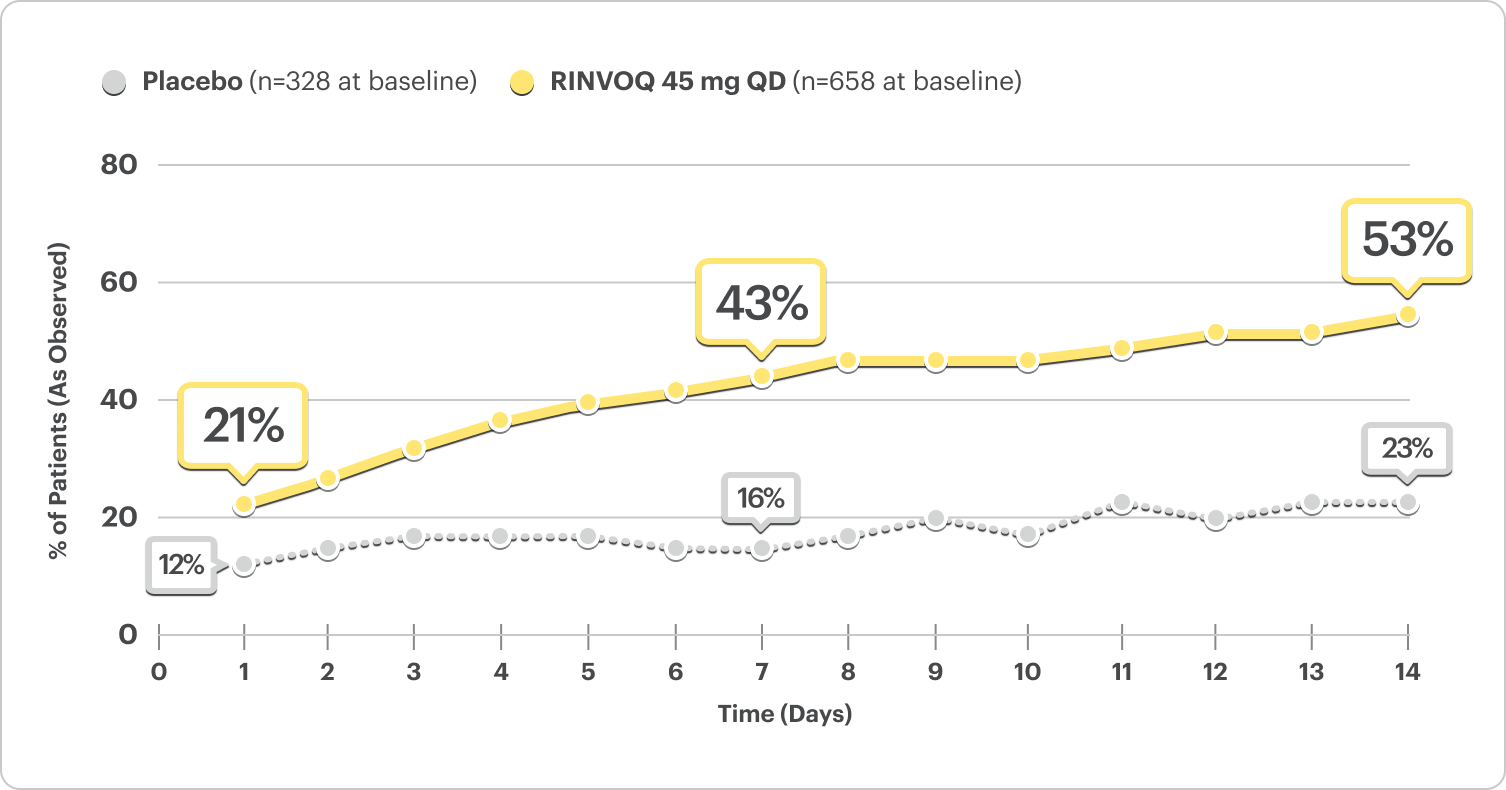
- At Day 1, 21% of patients on RINVOQ 45 mg reported a reduction in stool frequency vs 12% on placebo5
- At Week 1, 43% of patients on RINVOQ 45 mg reported a reduction in stool frequency vs 16% on placebo5
DATA LIMITATIONS: Post hoc analyses were not adjusted for multiplicity; thus, no statistical inferences can be made. These analyses utilized an as-observed approach and did not impute values for missing evaluations.
No Bowel Urgency Data by Day5
(Daily Patient Diary Data)
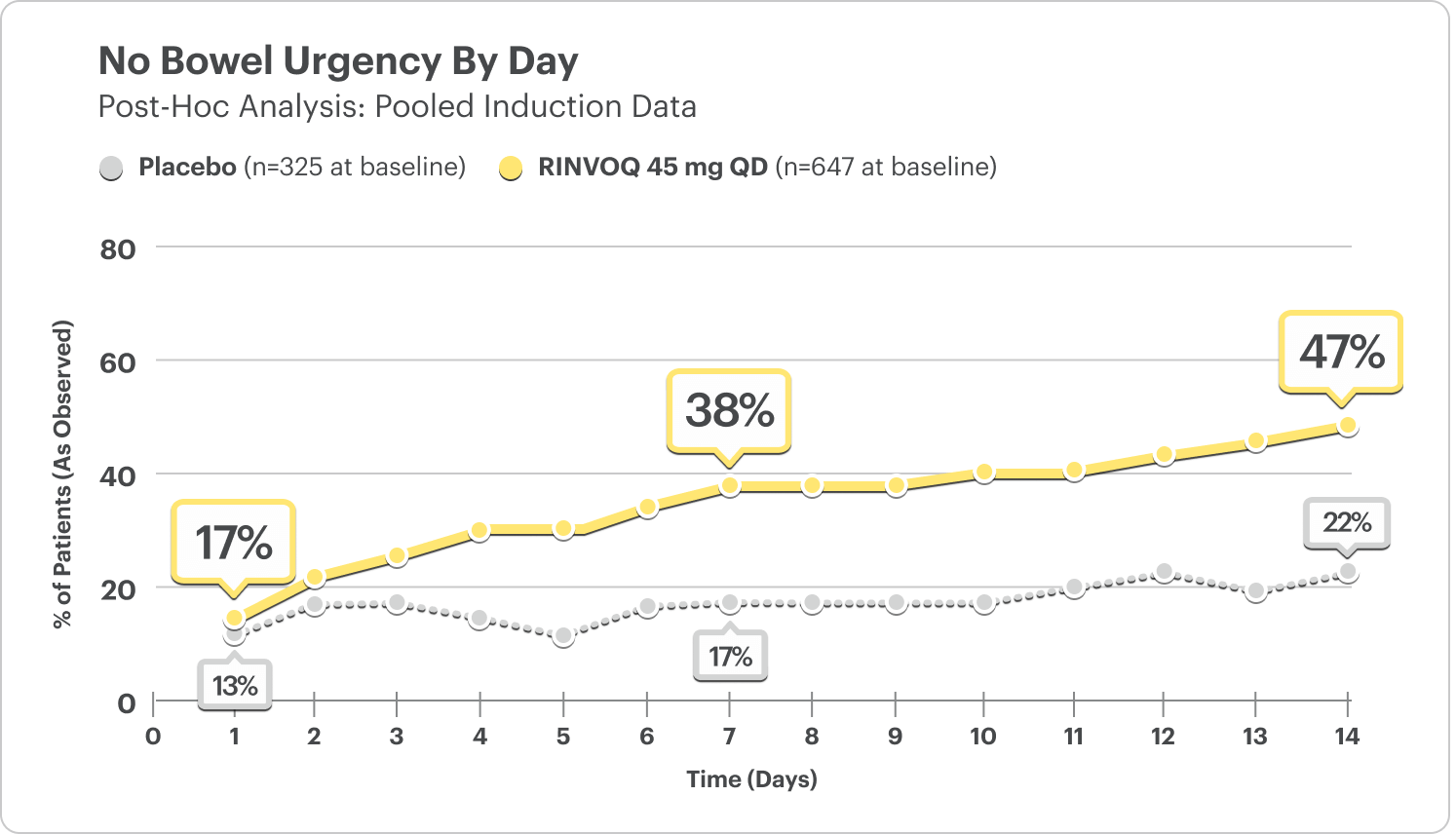
- At Day 1, 17% of patients on RINVOQ 45 mg reported no bowel urgency vs 13% on placebo.5
- At Week 1, 38% of patients on RINVOQ 45 mg reported no bowel urgency vs 17% on placebo.5
In the induction studies, a greater proportion of patients treated with RINVOQ 45 mg once daily compared to placebo had no bowel urgency (U‑ACHIEVE: 48% vs 21%, U‑ACCOMPLISH: 54% vs 26%) at Week 8.1
DATA LIMITATIONS: Post hoc analyses were not adjusted for multiplicity; thus, no statistical inferences can be made. These analyses utilized an as-observed approach and did not impute values for missing evaluations.
Fecal Calprotectin Reduction at Weeks 2 and 86
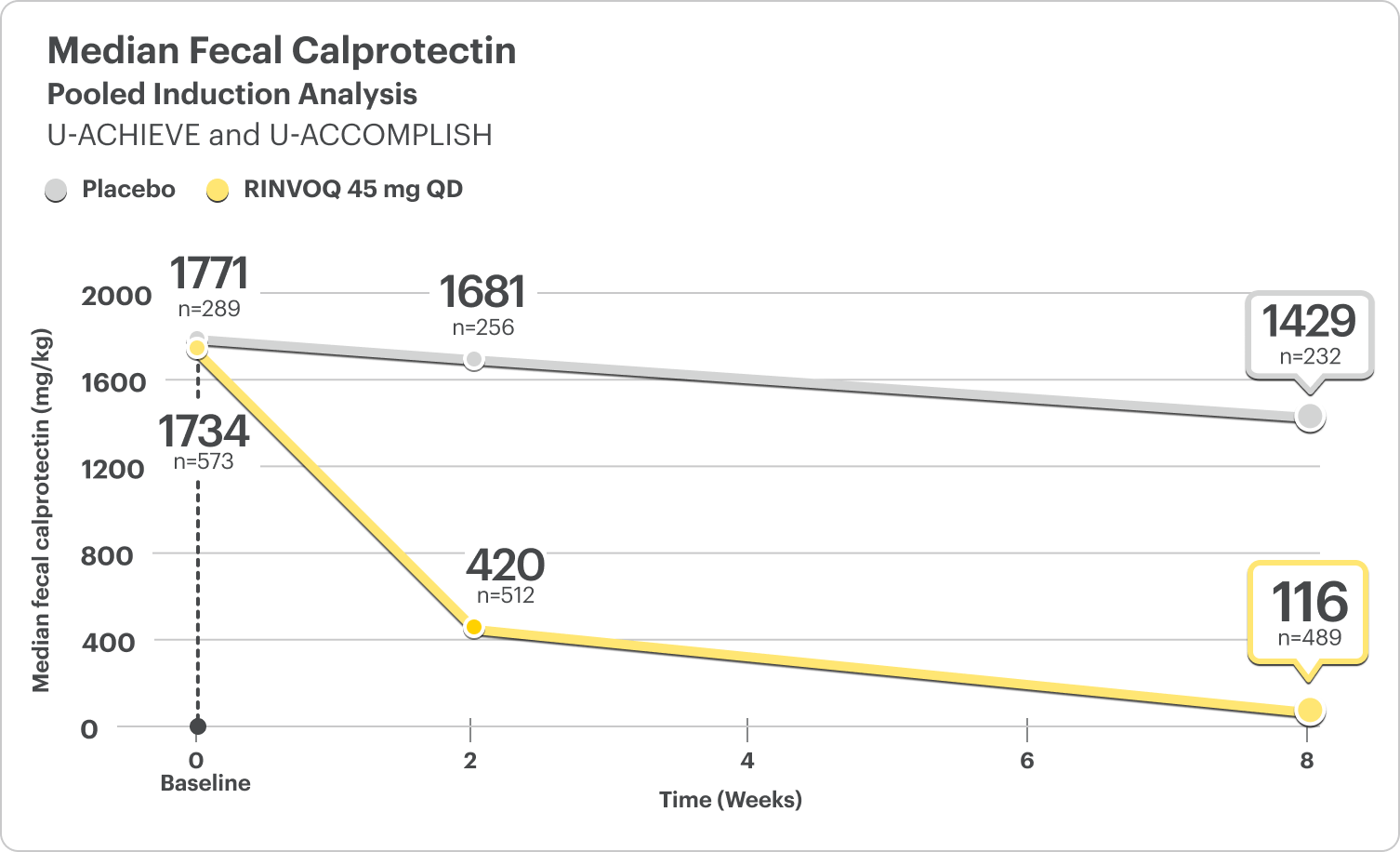
FECAL CALPROTECTIN <150 MG/KG
- At Week 2, 30% of patients on RINVOQ 45 mg had FC <150 mg/kg compared to 5% on placebo6
- At Week 8, 46% of patients on RINVOQ 45 mg had FC <150 mg/kg compared to 8% on placebo6
DATA LIMITATIONS: Fecal calprotectin <150 mg/kg at Week 2 and Week 8 of the integrated induction data was a prespecified analysis. Change from baseline in median fecal calprotectin at Week 2 and Week 8 was a post hoc analysis. Neither of these analyses were adjusted for multiplicity; thus, no statistical inferences can be made.

Interested in the safety data for RINVOQ?
See RINVOQ’s safety data across clinical trials