
AS and nr-axSpA Patients Met ASAS40 at Week 14 (Primary Endpoint) and Disease Control Through ASDAS Low Disease Activity at Week 14 (Ranked Secondary Endpoint) With Responses Observed at 2 Years1-6
Not an actual AS or nr-axSpA patient
AS=ankylosing spondylitis; ASAS=Assessment of SpondyloArthritis international Society; ASAS40=≥40% improvement and an absolute improvement from baseline of ≥2 units on a scale of 0 to 10 in at least 3 of the 4 domains, with no worsening in the fourth domain: total back pain, inflammation (mean score of BASDAI questions 5 and 6 on severity and duration of morning stiffness), physical function (BASFI), and patient global assessment of disease activity; ASDAS=Ankylosing Spondylitis Disease Activity Score; BASDAI=Bath Ankylosing Spondylitis Disease Activity Index; BASFI=Bath Ankylosing Spondylitis Functional Index; bDMARD=biologic disease-modifying antirheumatic drug; IR=intolerance or inadequate response; nr-axSpA=non-radiographic axial spondyloarthritis; TNFi=tumor necrosis factor inhibitor.
aRINVOQ is on a preferred tier or otherwise has preferred status on the plan’s formulary.
bCoverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
INDICATIONS
RINVOQ is indicated for the treatment of active ankylosing spondylitis (AS) in adults who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers.
RINVOQ is indicated for the treatment of active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation in adults who have had an inadequate response or intolerance to TNF blocker therapy.
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biologic disease-modifying antirheumatic drugs (bDMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine.
Exceptional Patient and Access Support
- ~99% preferred combined national commercial and Medicare Part D formulary coverage under the pharmacy benefit as of November 2025 in AS and nr-axSpA7,†,‡
- 1:1 support to help AS and nr-axSpA patients start and stay on track with their prescribed treatment plan
- Get patients started on RINVOQ Complete by downloading the enrollment form
*In RA: ~9.5 years maximum exposure (~4.2 years median) to RINVOQ 15 mg as of August 15, 2025. In PsA: ~6.4 years maximum exposure (~3.6 years median) to RINVOQ 15 mg as of August 15, 2025.8
†RINVOQ is on a preferred tier or otherwise has preferred status on the plan's formulary.
‡Coverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
AS=ankylosing spondylitis; ASAS=Assessment of SpondyloArthritis international Society; ASAS40=≥40% improvement and an absolute improvement from baseline of ≥2 units on a scale of 0 to 10 in at least 3 of the 4 domains, with no worsening in the fourth domain: total back pain, inflammation (mean score of BASDAI questions 5 and 6 on severity and duration of morning stiffness), physical function (BASFI), and patient global assessment of disease activity; ASDAS=Ankylosing Spondylitis Disease Activity Score; bDMARD=biologic disease-modifying antirheumatic drug; IR=intolerance or inadequate response; max.=maximum; nr-axSpA=non-radiographic axial spondyloarthritis; PsA=psoriatic arthritis; RA=rheumatoid arthritis; TNFi=tumor necrosis factor inhibitor.
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
Rapid Improvement in ASAS40 at Week 141,4,5
Responses observed as early as Week 4 (AS) and Week 2 (nr-axSpA)
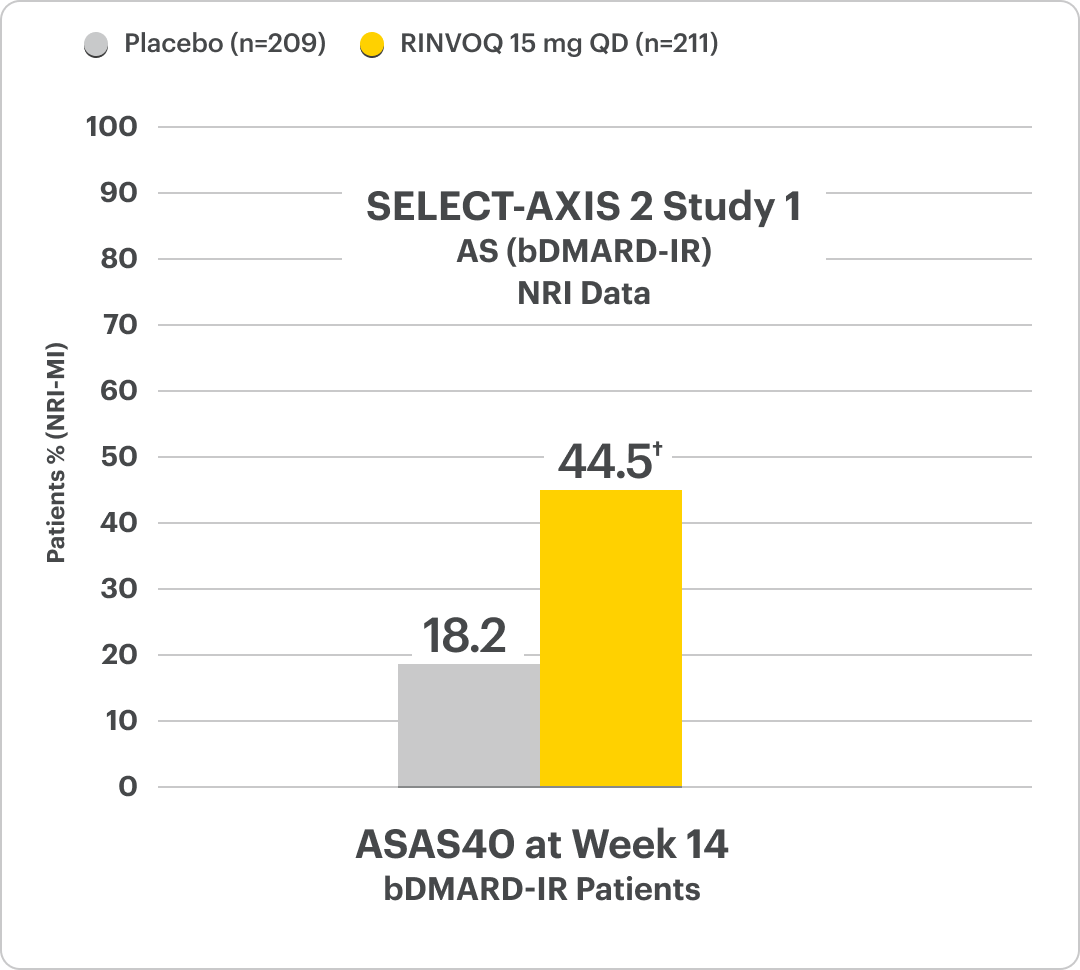
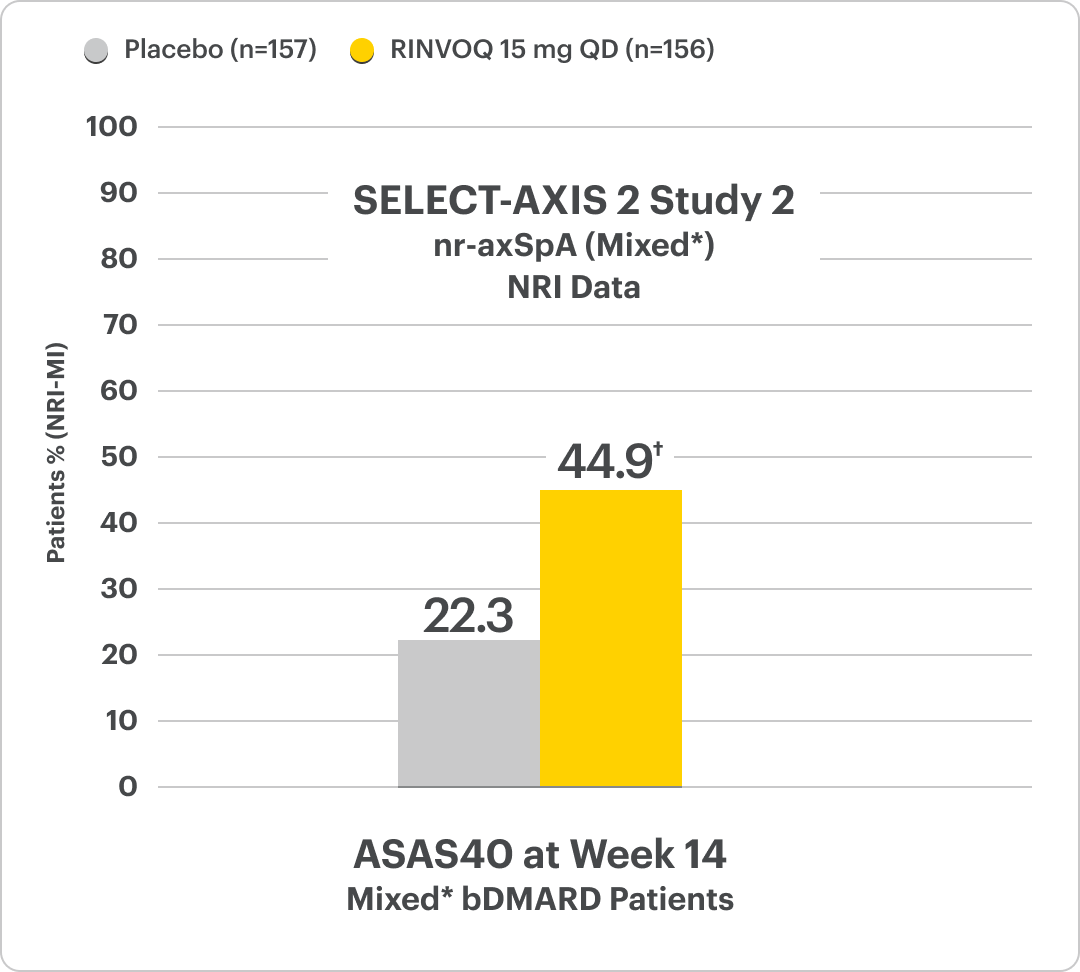
Significant ASAS40 Response vs Placebo at Week 141,4,5
RINVOQ is indicated for TNFi-IR patients.
Improvement in ASAS40 response observed at Week 4 (AS) and Week 2 (nr-axSpA):1,4,5
SELECT-AXIS 2 Study 1: AS: 22% RINVOQ 15 mg vs 12% placebo4,‡
SELECT-AXIS 2 Study 2: nr-axSpA: 12% RINVOQ 15 mg vs 6% placebo5,‡
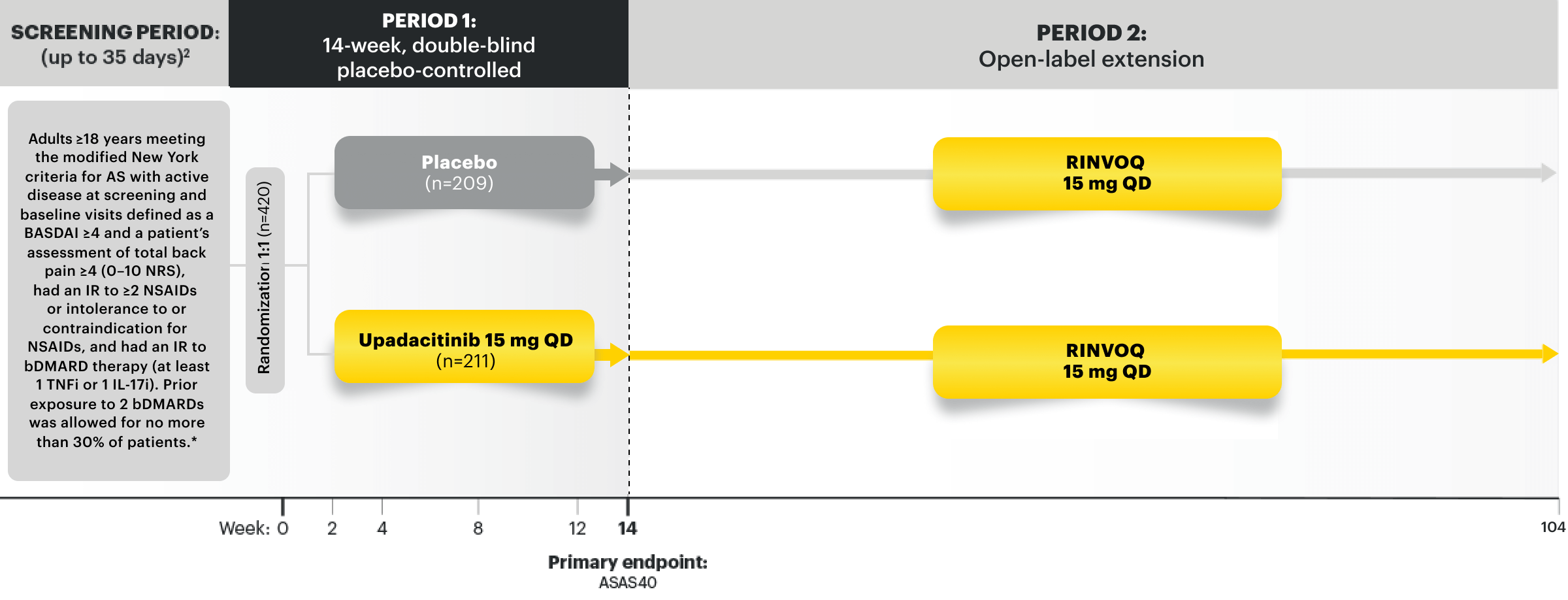
SELECT-AXIS 2 Study 1: AS Design Intro1,4:
14-week, double-blind, parallel-group, placebo-controlled, Phase 3 study of 420 patients with active AS who had an intolerance or inadequate response to at least 2 NSAIDs and 1 or 2 bDMARDs. Patients were randomized to receive RINVOQ 15 mg QD or placebo. Patients could continue background NSAIDs.
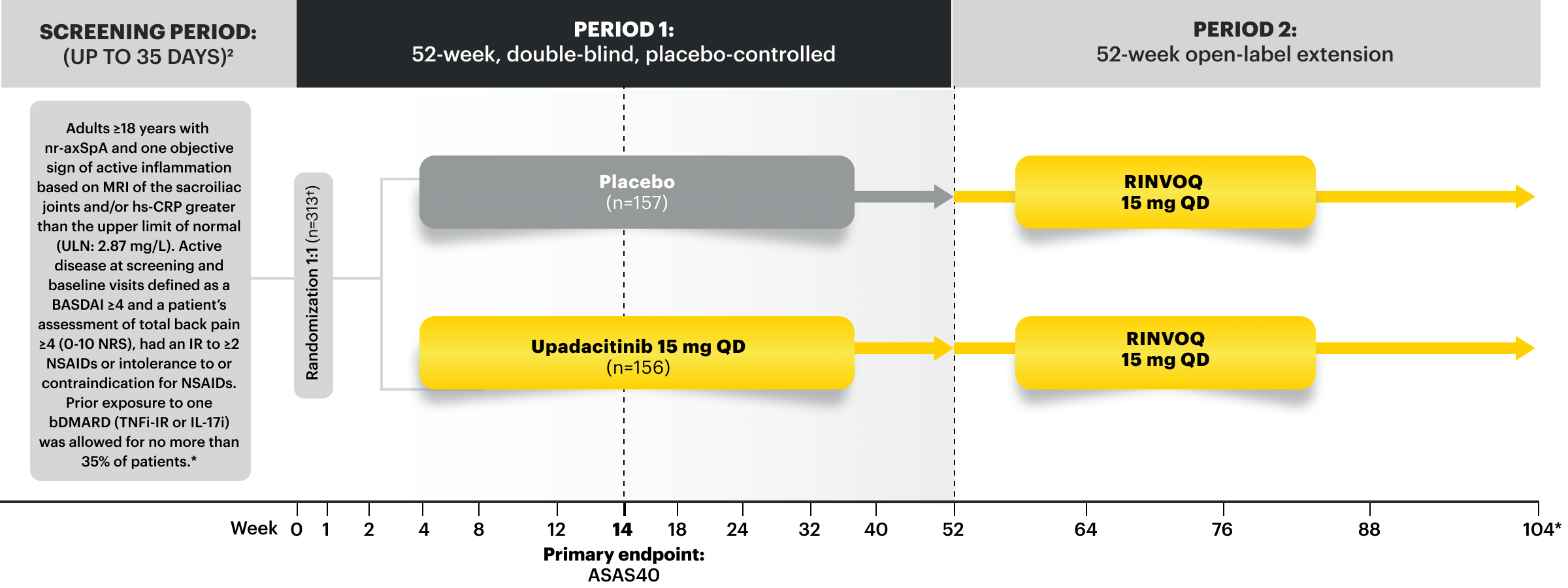
SELECT-AXIS 2 Study 2: nr-axSpA Design Intro1,5:
52-week, double-blind, placebo-controlled, Phase 3 study of 313 patients with nr-axSpA and 1 objective sign of active inflammation based on MRI of the sacroiliac joints and/or hsCRP greater than the upper limit of normal (ULN; 2.87 mg/L). Patients had an intolerance or inadequate response to at least 2 NSAIDs and, in 33%, to 1 bDMARD. Patients were randomized to receive RINVOQ 15 mg QD or placebo. Patients could continue background NSAIDs.
DATA LIMITATIONS: Data labeled as a primary endpoint at Week 14 were multiplicity-controlled. All other comparisons were not adjusted for multiplicity; therefore, statistical significance has not been established.
*Mixed=67% bDMARD-naïve & 33% bDMARD-IR5
†P<0.00014,5
‡P<0.05; P-value obtained through nominal statistical testing4,5
AS=ankylosing spondylitis; ASAS=Assessment of SpondyloArthritis international Society; ASAS40=≥40% improvement and an absolute improvement from baseline of ≥2 units on a scale of 0 to 10 in at least 3 of the 4 domains, with no worsening in the fourth domain: total back pain, inflammation (mean score of BASDAI questions 5 and 6 on severity and duration of morning stiffness), physical function (BASFI), and patient global assessment of disease activity; BASDAI=Bath Ankylosing Spondylitis Disease Activity Index; BASFI=Bath Ankylosing Spondylitis Functional Index; bDMARD=biologic disease-modifying antirheumatic drug; COVID-19=coronavirus disease-2019; hsCRP=high-sensitivity C-reactive protein; IR=intolerance or inadequate response; MRI=magnetic resonance imaging; nr-axSpA=non-radiographic axial spondyloarthritis; NRI=nonresponder imputation; NRI-MI=nonresponder imputation incorporating multiple imputation to handle missing data due to COVID-19; NSAID=nonsteroidal anti-inflammatory drug; QD=once daily; TNFi=tumor necrosis factor inhibitor; ULN=upper limit of normal.