
PsA patients met ACR20 at Week 12 (Primary Endpoint) and Disease Control through Minimal Disease Activity (MDA) at Week 24 (Ranked Secondary Endpoint), observed up to ~3 years.1-3
ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria (patient assessment of pain, patient global assessment, HAQ-DI, physician global assessment, and hs-CRP); IR=intolerance or inadequate response; TNFi=tumor necrosis factor inhibitor.
aRINVOQ is on a preferred tier or otherwise has preferred status on the plan’s formulary.
bCoverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
INDICATION
RINVOQ is indicated for the treatment of adults with active psoriatic arthritis (PsA) who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers.
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biologic disease-modifying antirheumatic drugs (bDMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine.
Exceptional Patient and Access Support
- ~99% preferred combined national commercial and Medicare Part D formulary coverage under the pharmacy benefit as of May 2025 in PsA4,†,‡
- 1:1 support to help PsA patients start and stay on track with their prescribed treatment plan
- Get patients started on RINVOQ Complete by downloading the enrollment form
Rapid Relief3
- ACR20 response seen as early as Week 2
Durable Control3,4
Response Rates of:
- MDA at Week 24
- Joint Efficacy (ACR20/50/70) at Week 12
- Skin Efficacy (PASI 75) at Week 16 with data observed up to ~3 years
RINVOQ is not indicated for the treatment of plaque psoriasis
Well-Studied Safety6
~5.0 years max. exposure in PsA (~2.9 years median) to RINVOQ 15 mg as of 08/15/22
Exceptional Access and Patient Support
- >95% preferred national commercial and Medicare Part D formulary coverage under the pharmacy benefit as of 2
- 1:1 support to help PsA patients start and stay on track with their prescribed treatment plan
- Get patients started on RINVOQ Complete by downloading the enrollment form
*In RA: ~8.5 years maximum exposure (~4.2 years median) to RINVOQ 15 mg as of 08/2024. In AS: ~3.8 years maximum exposure (~1.8 years median) to RINVOQ 15 mg as of 08/2024. In nr-axSpA: ~2.3 years maximum exposure (~1.0 year median) to RINVOQ 15 mg as of 08/2024.5
†RINVOQ is on a preferred tier or otherwise has preferred status on the plan's formulary.
‡Coverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria (patient assessment of pain, patient global assessment, HAQ-DI, physician global assessment, and hsCRP); AS=ankylosing spondylitis; nr-axSpa=non-radiographic axial spondyloarthritis; PsA=psoriatic arthritis.
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
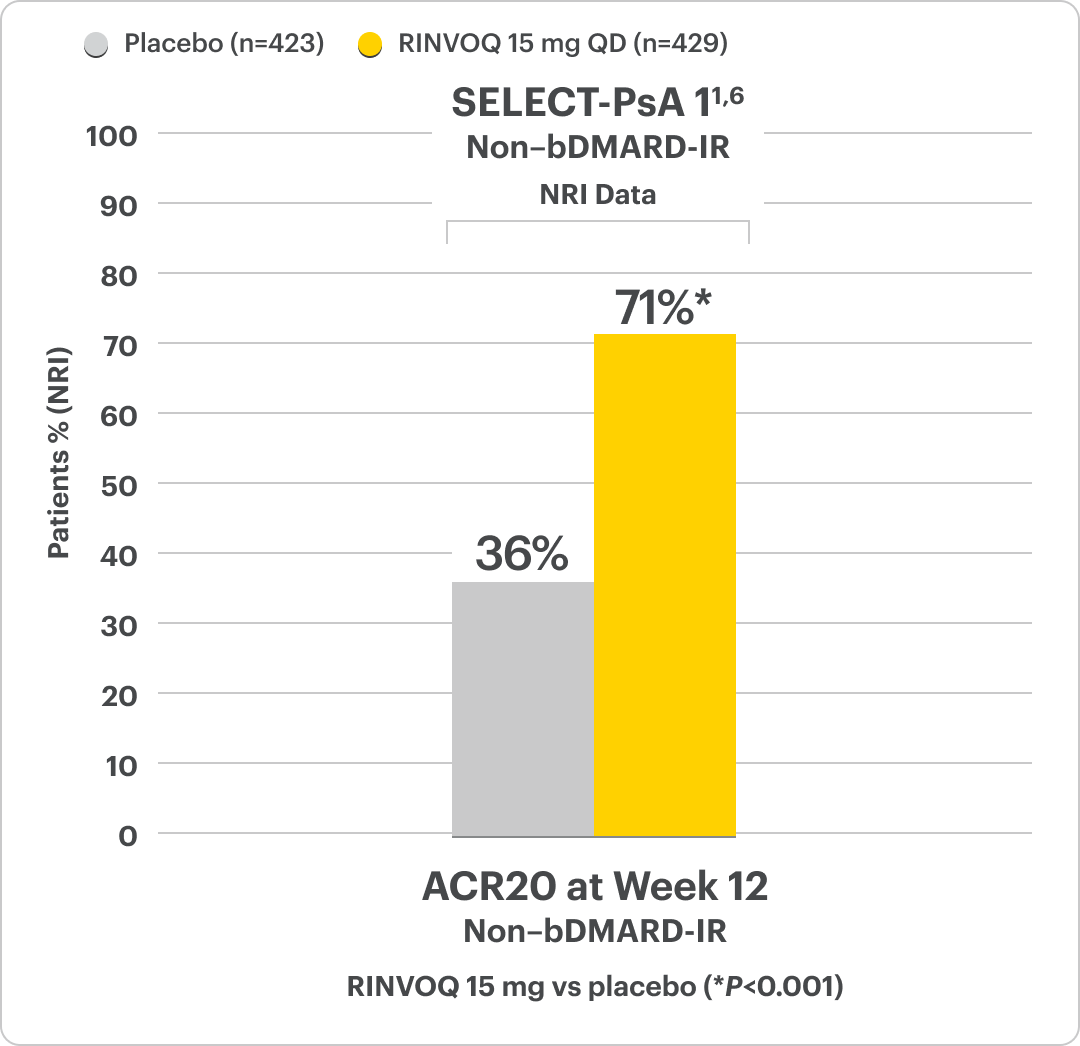
RINVOQ Met Its Primary Objective, ACR20 Response, in 2 Clinical Studies1
Significant ACR20 Response at Week 12 (Primary Endpoint) vs Placebo1,2,6
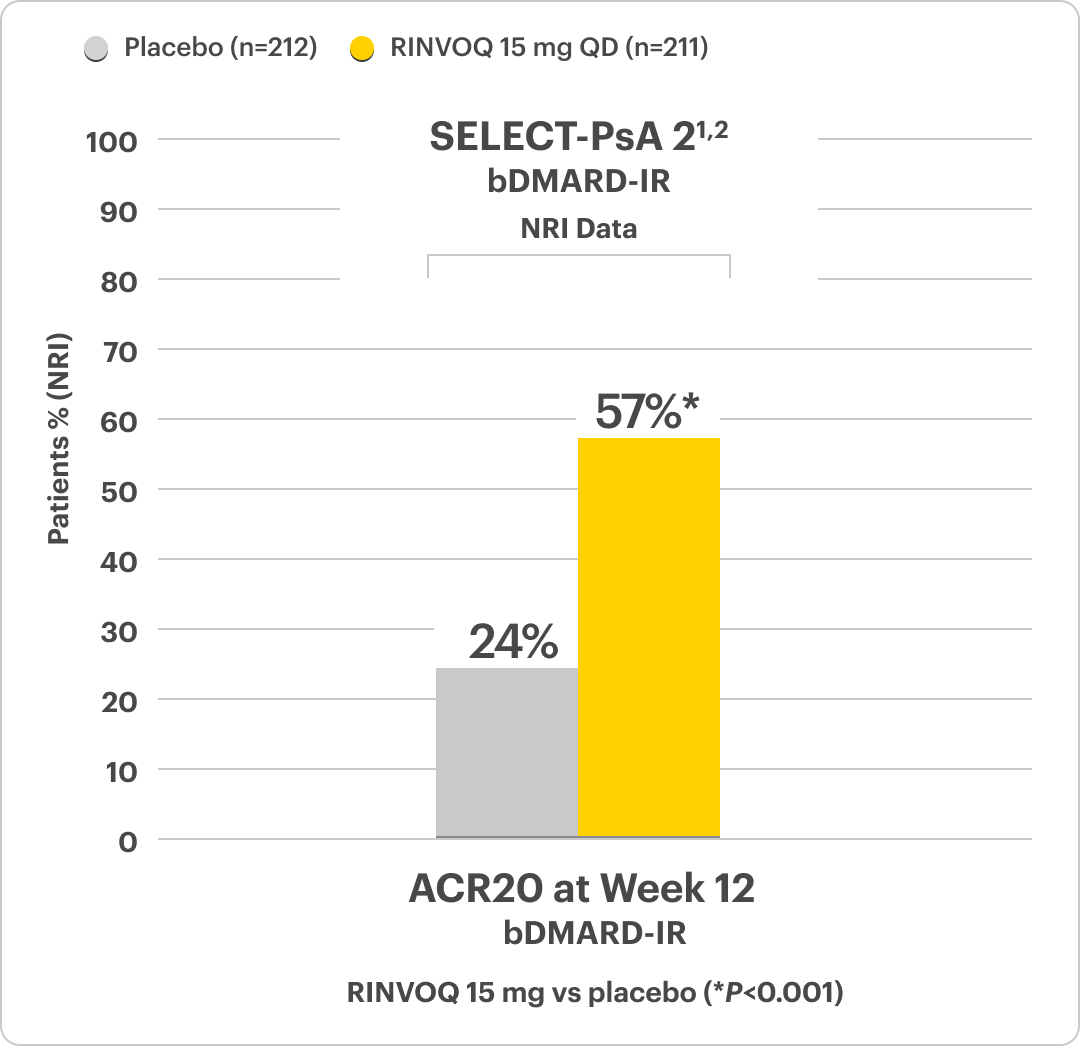
RINVOQ is indicated for TNFi-IR patients.
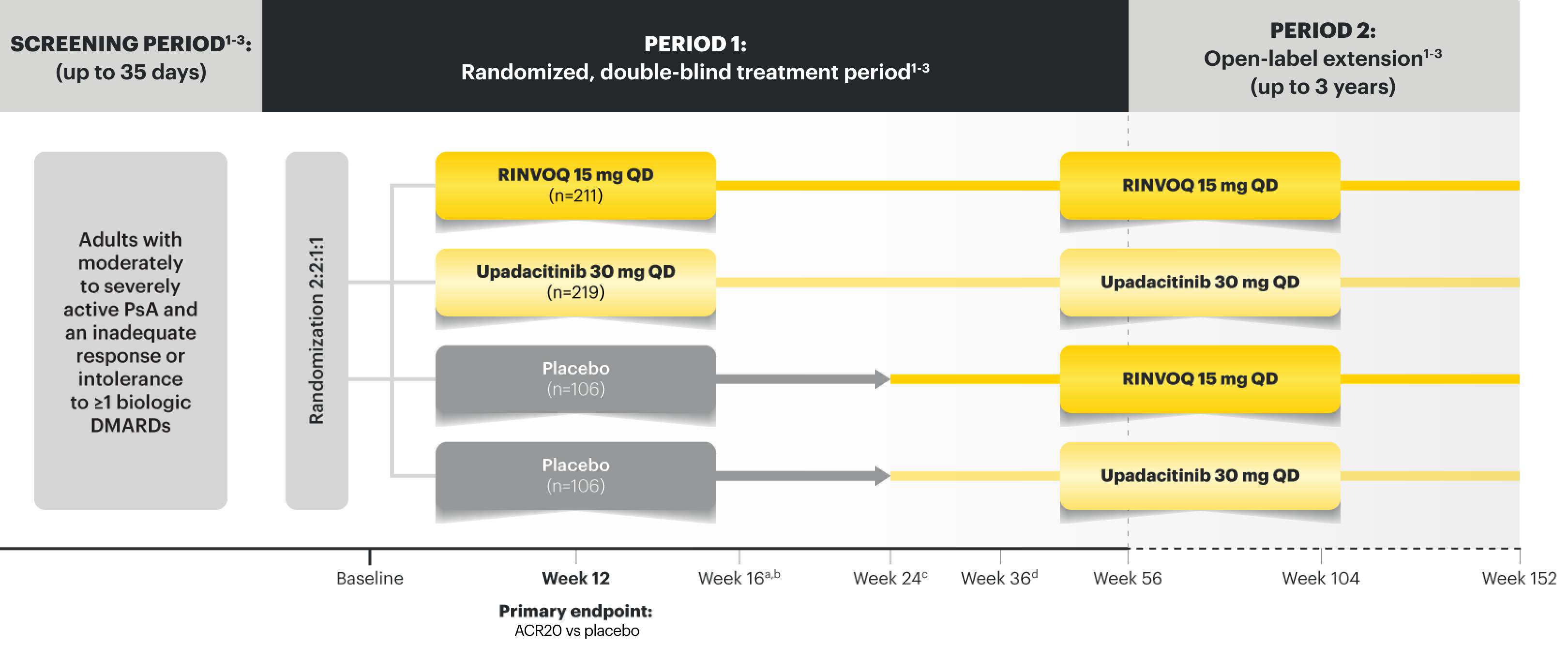
SELECT-PsA 2 Study Design Intro1,2:
24-week, double-blind, placebo-controlled study of 642 adult patients with moderately to severely active PsA who had an inadequate response or intolerance to at least 1 biologic DMARD. Patients were randomized to receive upadacitinib or placebo. The primary endpoint was proportion of patients achieving ACR20 response at Week 12 vs placebo.
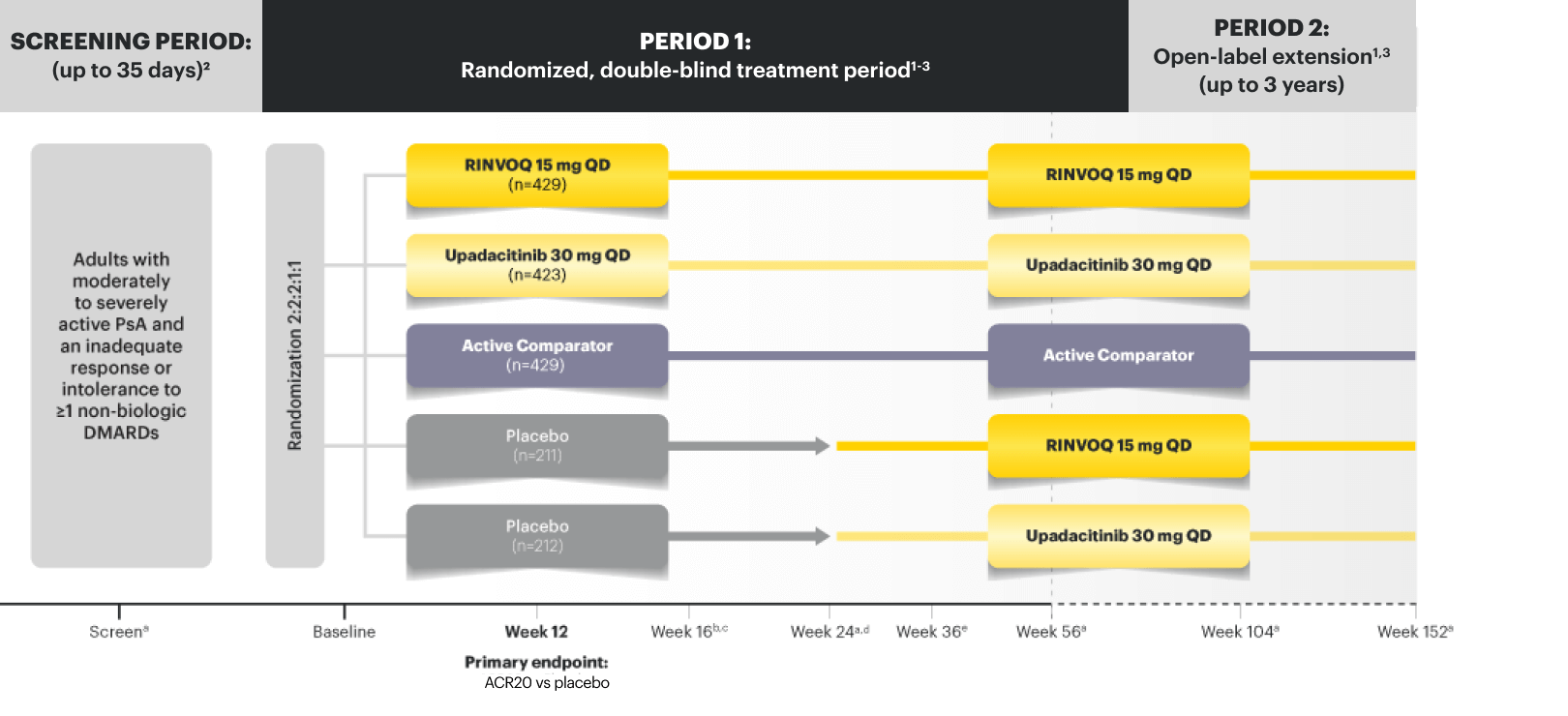
SELECT-PsA 1 Study Design Intro1,6:
24-week, double-blind, placebo-controlled study of 1705 adult patients with moderately to severely active PsA who had an inadequate response or intolerance to at least 1 non-biologic DMARD. Patients were randomized to receive either upadacitinib, active comparator, or placebo. The primary endpoint was proportion of patients achieving ACR20 response at Week 12 vs placebo.
Rapid ACR20 Response Seen as Early as Week 22
SELECT-PsA 2: 33% RINVOQ 15 mg vs 11% placebo (NRI)2
DATA LIMITATIONS: Week 2 data for all comparisons were not adjusted for multiplicity; therefore, statistical significance has not been established.
ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria (patient assessment of pain, patient global assessment, HAQ-DI, physician global assessment, and hsCRP); DMARD=disease‑modifying antirheumatic drug; IR=intolerance or inadequate response; NRI=nonresponder imputation; PsA=psoriatic arthritis; QD=once per day; TNFi=tumor necrosis factor inhibitor.