Dr. Adam Brown
At the crossroads of clinical research and in-clinic experience, this is The Breakout Rheum. Join me, Dr. Adam Brown, as leading rheumatologists come to the table with their take on trending data. This podcast is brought to you by AbbVie.
Welcome to The Breakout Rheum podcast—R-H-E-U-M. I’m your host, Dr. Adam J. Brown, and today, we’ll be talking about giant cell arteritis, or GCA, as the cool kids call it.
In this episode, we will focus on remission and the use of glucocorticoids and also review some clinical data for a steroid-sparing treatment option. Before we start, let me introduce a colleague of mine and our guest for today, Dr. Anisha Dua, who is a rheumatologist and vasculitis expert. Dr. Dua, thank you so much for being here.
Dr. Anisha Dua
Thank you, Adam, it’s a pleasure to be here. I’m really excited to be here with you talking about GCA.
Dr. Adam Brown
So, Anisha, I know you well, but for all listeners, can you tell them a bit about yourself and your practice?
Dr. Anisha Dua
Yeah, I’d be happy to. So, I am a professor of medicine, and I direct the Vasculitis Center and the Rheumatology Fellowship training program here at Northwestern Medicine in Chicago, Illinois. I’m from Pittsburgh—go Steelers—and I worked at Allegheny Health
Network in Pittsburgh for a while before going to the University of Chicago and then joining Northwestern around six years ago.
And I’ve been practicing with a focus on really multidisciplinary management of vasculitis patients since I graduated fellowship in 2012. My research interests are really in therapeutics and management of vasculitis patients and really in medical education. I’ve got a lot of patients, about probably 700 with vasculitis on my panel, and probably around 1- or 200 with GCA and PMR. So, I know that you and I have some overlapping interests, like in engaging learners in medical education and history.
That was actually my major in college. And talking about, as well as taking care of, patients with vasculitis.
Dr. Adam Brown
So, I agree, we do have multiple overlapping interests, including medical history and vasculitis. But I have to say our football interests are divergent. I am a Cleveland Browns fan, but!
Dr. Anisha Dua
We won’t get into that here in today’s podcast.
Dr. Adam Brown
It would be too painful for me. Yes, I agree, let’s not talk about it. So, I feel like history and especially history of medicine is fantastic. And rheumatology, in particular, is a great field for that because we have so many mysteries in rheumatology. And I think learning the history of how people kind of figured out these diseases, and the pathophysiology behind these diseases really kind of helps understand the field as best we can.
So, as I mentioned, we have history of vasculitis in common. Another thing we have in common is that we were both practicing in cities that start with a C, if you can believe it!
Dr. Anisha Dua
I can.
Dr. Adam Brown
Yeah. You’re in Chicago and I am in Cleveland. I’ve been practicing at the Cleveland Clinic for about ten years, and prior to that I was at Washington, DC.
I was practicing in Georgetown. So, let’s jump to the topic of GCA. What do you think is important for our listeners to know about the disease?
Dr. Anisha Dua
Well, GCA is a large vessel vasculitis. It can cause inflammation of the aorta and its branches. And this leads to ischemia and damage. It’s actually the most common type of vasculitis and it affects the older age population. Now some of the classic features include things like headache, jaw claudication—and that’s important to differentiate from TMJ. It’s really more of a soreness that occurs with chewing and resolves when you stop.
So, it develops and builds up over time, not just pain when you open your jaw really wide. Other features include constitutional symptoms: fevers, fatigue, weight loss, just generally feeling unwell. But the most feared complication is vision loss. And that can happen abruptly. It’s often not reversible. So, you know these are some severe symptoms. They can be very painful and really concerning for patients.
Many of them, if they’re not already being managed by a rheumatologist, they might go to the E.R. and then get referred to us. And our job as a rheumatologist is to assess these patients when there is suspicion of GCA as quickly as we can. And that’s why we are often moving our schedules around to try to fit them in.
These patients are started on glucocorticoids right away, and then confirmation of GCA can be done in various ways. The most common diagnostic method used by rheumatologists is the temporal artery biopsy, especially here in the US. But ultrasound and imaging of the large blood vessels is something that I do, and it’s becoming more common, which is really important and a good thing.
Dr. Adam Brown
I agree, GCA is definitely one of the more severe diseases that we manage as rheumatologists, and one that can have long-lasting consequences if it’s not recognized and treated right away. And I think it’s important to recognize the heart of today’s conversation is around getting patients with GCA into remission. I know that for me, a part of the journey to remission means that we are reducing the ongoing inflammation and prevent complications like vascular ischemic damage, avoiding the dreaded complication of vision loss.
So, Anisha, how do you define remission in GCA?
Dr. Anisha Dua
Well, Adam, that’s a really important question. I mean, based on ACR guidelines, remission is defined as the absence of signs and symptoms of GCA. In clinical practice, what that means is, you know, resolution of the headaches, the constitutional symptoms like jaw claudication, PMR symptoms, all this stuff I mentioned, but also importantly, no new or evolving symptoms. Now, large vessel involvement can be a little harder to measure in terms of clinical remission.
And it’s important to remember that once damage has been done by the inflammation, we really can’t reverse it. So, some patients may have residual deficits from the disease, as well as side effects from the med used to treat it. And that doesn’t necessarily mean that they’re not in remission. So, if there is evidence of large vessel involvement at baseline, I do tend to monitor these patients with imaging studies every six months.
Or of course, if there’s a new symptom that comes up and if they’re in remission or once they’re completed on treatment, I’ll try to space out the imaging studies. But complications can occur. And those include thoracic and aortic dissections and aneurysms. And those carry a high morbidity and mortality, so those are worrisome. And this can happen years after the disease onset.
So, I do continue to monitor these patients long term. I always tell my patients once they have this diagnosis, they’re going to be a friend of mine for a long time. I will be monitoring them long term. The other thing that I always check with these folks is inflammatory markers. Now, there’s no perfect biomarker for this disease or many of our diseases.
And these are not specific to GCA. Patients can be in remission clinically with elevated inflammatory markers. Another important thing to remember is that these values can be higher in elderly patients or based on other comorbidities. Despite those drawbacks, I do tend to check them regularly, especially as I’m trying to wean down glucocorticoids.
Dr. Adam Brown
I like what you said earlier about resolution of symptoms in these patients, once steroids are started. I think it’s really satisfying as a rheumatologist, when these patients come in and the diagnosis is made and steroids are started, and you can see the resolution of the headache, the resolution of the jaw claudication as you described. And then kind of that pain, that stiffness these patients often have, when steroids are started, they just kind of all resolve really dramatically and can be very satisfying.
And the patient can really like you after that. So, let’s talk a little bit more about that last point you made. You said you monitor these patients, especially as you’re trying to wean down the steroids. And you’d also mentioned earlier how important it is to start the steroids right away. What are your thoughts about the role of steroids in treating GCA?
What does it mean to wean them and what considerations stay top of mind when prescribing these to the older patient population in GCA?
Dr. Anisha Dua
I think it’s really important for our listeners to understand—it’s important that we start our patients on steroids immediately to reduce that inflammation and prevent complications such as vision loss. So, for me, when I prescribe steroids, especially at high doses or for longer than a week or two, I consider the various risk factors that a patient has and age is one of them.
So, for an older patient with GCA, I’m thinking about their comorbid conditions, right? Diabetes, prior fractures, cataracts, psychiatric history. And all of these will weigh into my treatment plan. Now, historically, we really didn’t have any other options outside of steroids. And I had a lot of difficulty trying to keep my patients on steroids. Many of them would come in just really frustrated.
So many complications from their diabetes, new fractures, weight gain, just a ton of side effects. And we know they’re associated with glucocorticoids, but I really had nothing else to offer. So, I’d be telling them, you know, you’re going to be on this for a year, if not two or more, depending on your clinical course. And those are really tough conversations to have with your patients.
But now, luckily, with the evolving landscape and GCA management, we’ve got options. And that lets us get our patients off of steroids much quicker than ever before. And I’ve been able to get some of my patients off of steroids within six months with the use of advanced therapies.
Dr. Adam Brown
I agree, the advancements in care have allowed us to help get our patients off steroids, which is really important. Whenever I’m talking to a patient about steroids, I’m kind of talking about the acute effects of steroids and the long-term effects of steroids, and then the potential inevitable side effects, long term of being on higher doses for long periods of time.
I talked to patients about how their blood pressure may rise early on, how their blood sugars may rise very early on, how also their sleep can be affected, their mood could be affected. I can kind of help, especially when I’m seeing a patient in a hospital and they’re getting high-dose steroids. I say, hey, you might be like staring at the ceiling at three in the morning and really upset with me because they can’t sleep.
But then the long-term effects of these steroids, as you mentioned, they can be on this for 1 or 2 years or more. I kind of talk about bone health in these patients, blood pressure monitoring, how to kind of watch for their blood sugars and potentially increasing the risk of long-term diabetes and, of course, opportunistic infections.
So, can you share with us, Anisha, the kind of things that you’ve seen?
Dr. Anisha Dua
Yeah. You know, at home, a lot of the things you’re mentioning, these are all stuff that we have to talk about and be upfront with, with our patients. Not everyone’s going to get every side effect, but pretty much everyone’s going to get something, right? And so, like I mentioned, I specialize in vasculitis since 2012. And back then, steroids really were the only option.
So, I’ve seen, unfortunately, a ton of these sort of situations. I think some of the most common ones that are really frustrating are ones where like patients would be on steroids for a long period of time. I was unable to get their disease under control. I kept trying to wean it, but they’d have active disease, so they’d be just on these steroids for ages and ages.
Underlying diabetes. They’d come in with, you know, worsening blood sugars, but also retinopathy, wounds that were developing from their diabetes. And then on top of that, infections, which we know steroids make worse as well. So, it’s sort of this vicious cycle where they’d have the steroids for the treatment of their GCA and then all these complications like diabetes or hip fractures, you know, and now they’re immobilized and becoming more and more weak.
And just one thing leads to another. The other thing that happens is patients, you know, aren’t sleeping. It leads to a lot of interpersonal issues. They’re feeling uncomfortable. They’re gaining weight. They feel a little more debilitated. A lot of them often can’t like work the way they used to, function the way they used to, the fatigue can be overwhelming.
And so that leads to a lot of personal and interpersonal issues. And I’ve seen that happen with my patients as well. So, of course as a rheumatologist, with the patient’s health the top of mind, I always try to stop the steroids when I can, when it’s reasonable because of all these risks we talked about, particularly in these older patients with GCA.
But as you know, these conversations require a lot of shared decision-making with patients.
Dr. Adam Brown
Yeah, I agree, I find that my conversation with patients can be unique, depending on how they may have responded to the steroids and what type of side effects they’re experiencing. Like if a patient comes in: look at my face, look at my face, I’ve gained so much weight. A lot of patients know that term, the moon face, right?
They really want to get off these things as fast as possible because as you kind of alluded to, these have every side effect in the book. And they will say, what can I do to get off these steroids as quickly as possible? They may not be sleeping. They may be upset because now they’re on all these other medications to treat another medication.
Often their spouses and loved ones are very aware of the changes caused by the prednisone, and that’s why some of the patients are begging me to stop the steroids, while others may be a little bit more hesitant.
Dr. Anisha Dua
Yeah. Adam, a lot of times it’s the patients that have responded well to the steroids that are hesitant to be steroid-free. That’s where it’s important that we spend time educating them about the risks and benefits of ongoing steroid therapy, and how our goal is to get them into steroid-free remission. I had a patient recently where they did have vision loss due to the inflammation, and they were really fearful of new-onset vision loss in the other eye, so they were super hesitant for me to stop the steroids.
But they also had diabetes. So, you know, I had to talk to them and say, listen, there are possible ways to lose your vision from staying on these steroids. The complications from your diabetes, like glaucoma or formation of cataracts, those also can cause vision loss, and also your diabetes has been really hard to control. So, you know, keeping you on these steroids is going to lead to lots of complications outside of ocular issues.
But it is a really tough conversation to, to have. And you have to really take into consideration what the patient’s thinking about. I also have to, on another note, remind patients that even on low doses of steroids, adrenal insufficiency is a risk.
Dr. Adam Brown
Yeah, absolutely. I think you made a good point about, you know, especially the patients with the more severe manifestations of GCA can be the most hesitant to go off these steroids. So, I think that’s another thing we have in common. We agree that the goal of GCA is patients to be not just in remission, but actually, steroid-free remission.
Dr. Anisha Dua
Yeah. And I think that’s why all rheumatologists should be really excited about steroid- sparing therapies that can help our patients get into remission, stay in remission, and not suffer from this multitude of side effects that we’ve been talking about, about steroids. We know that targeting the IL-6 pathway can help some patients. However, there’s still a large unmet need.
And why I know that we are both excited about having another mechanism of action available.
Dr. Adam Brown
Yes, you’re right. As rheumatologists, we love mechanisms of action and targeting various cytokines. It is what we do. So, let’s talk about RINVOQ, or upadacitinib, which is a once- daily oral Janus kinase inhibitor indicated for the treatment of adults with giant cell arteritis.
Dr. Anisha Dua
What our listeners may not know is that with GCA indication, RINVOQ is now approved for a total of nine indications in immunology. RINVOQ is part of the JAK class and RINVOQ is not recommended for use in combination with other Janus kinase inhibitors, biologic disease- modifying antirheumatic drugs, or with potent immunosuppressants such as azathioprine and cyclosporine.
And of course, RINVOQ is contraindicated in patients with a hypersensitivity to RINVOQ or its excipients. RINVOQ is approved for other diseases that we treat in rheumatology. So, I’ve been using it for the past five years or so. I feel like I’ve developed a good sense of where RINVOQ is appropriate and where I’m comfortable prescribing it.
Dr. Adam Brown
I feel the same way. RINVOQ has a BOXED WARNING that includes Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thromboses, with additional warnings and precautions including Hypersensitivity Reactions, Gastrointestinal Perforations, Lab Abnormalities, Embryo-Fetal Toxicity, Vaccinations, and medication residue in stool. Please continue listening for additional important safety information within and at the end of this podcast.
Dr. Anisha Dua
So now let’s talk about the GCA study for RINVOQ.
Dr. Adam Brown
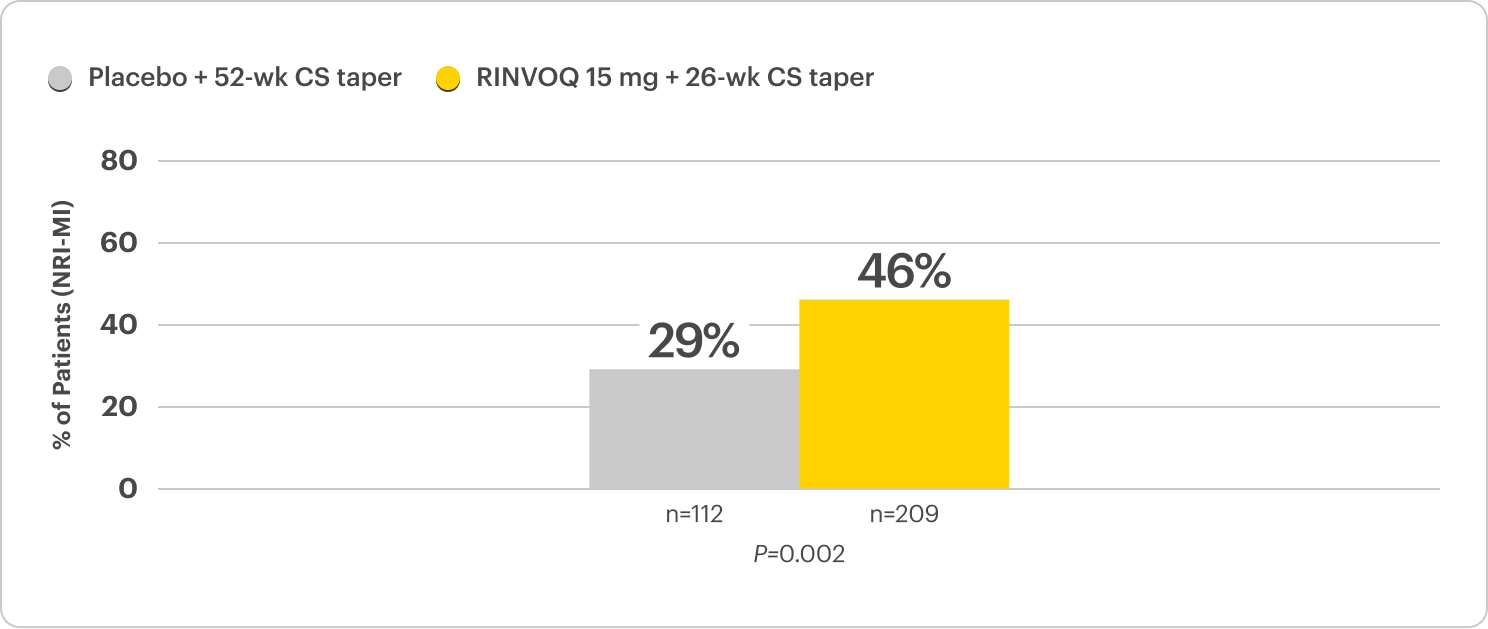
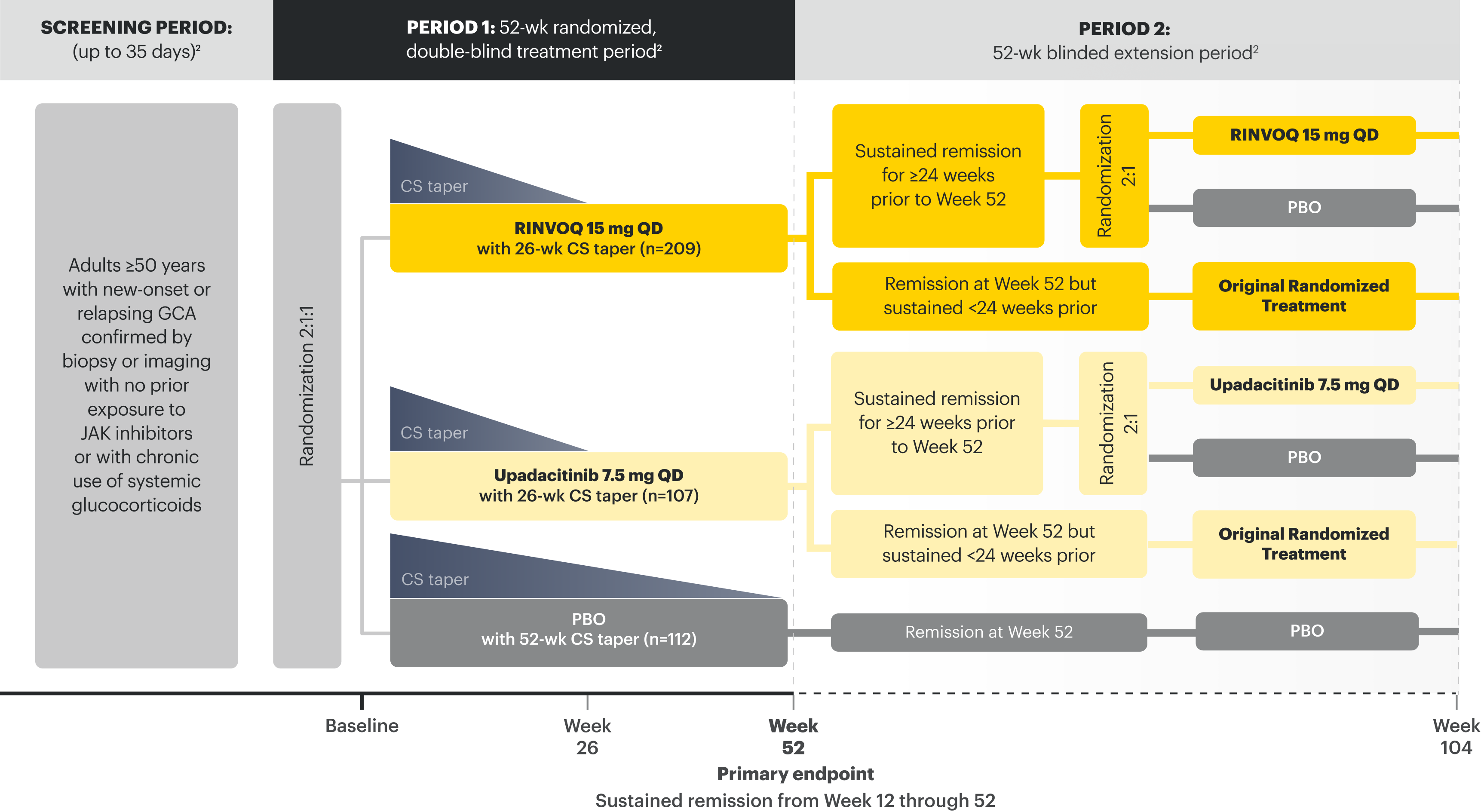
Sure. RINVOQ was studied in a Phase 3, multicenter, double-blind, placebo-controlled trial called SELECT-GCA. Patients enrolled were 50 years of age or older, and the mean age of patients was 71. It’s worth noting that this study began in 2017 and enrolled 428 patients, making it the largest clinical trial to date for GCA. Patients were assigned to treatment with two separate steroid-sparing regimens.
209 patients received RINVOQ 15mg in combination with steroids tapered off at 26 weeks. We will call that the RINVOQ arm. Meanwhile, 112 patients received placebo in combination with steroids tapered off at 52 weeks. We’ll call that the placebo arm. 107 patients received upadacitinib 7.5mg in combination with steroids tapered off at 26 weeks. Keep in mind that this is not the approved dose in GCA, so we’ll only review the data from the RINVOQ 15mg dose.
The RINVOQ arm met its primary endpoint, which was the percentage of patients achieving sustained remission from Week 12 through 52, defined as the absence of GCA signs and symptoms and adherence to the protocol-defined steroid taper regimen. 46% of patients in the RINVOQ arm achieved sustained remission compared to 29% of the patients in the placebo arm.
Dr. Anisha Dua
Those are great results. It’s amazing to hear that patients who received RINVOQ and followed a 26-week steroid taper achieve significantly higher rates of sustained remission compared to placebo patients who were on a 52-week steroid taper. Sustained remission is such an important outcome in GCA patients, because we all have had patients where they seem to respond to therapy and get better, but then either have full blown relapses or have some persistent grumbling disease that makes us just reach for more steroids.
And sometimes when we’re starting to wean those steroids at those lower doses, you know, patients’ symptoms, just, they just start creeping back up. The fact that these patients did not have or receive additional steroids above the protocol and were able to stay in remission is really important.
Dr. Adam Brown
Absolutely. So, let’s get into steroid-free remission, which we had agreed on is a goal for us and our patients. A key secondary endpoint in the SELECT-GCA trial was complete remission at Week 52. Complete remission was defined as the absence of GCA signs and symptoms and the adherence to the protocol-defined steroid taper and the normalization of key inflammatory markers.
ESR to less than 30mm per hour and high-sensitivity CRP to less than one milligram per deciliter. What we saw at Week 52 is that 50% of patients in the RINVOQ arm achieved complete remission, compared to 20% of patients in the placebo arm.
Dr. Anisha Dua
So essentially, this tells us that half the patients in the RINVOQ arm were able to come completely off steroids by Week 26, didn’t require any additional steroids through Week 52 to keep their disease well controlled, with normalization of their lab parameters at Week 52. The ability to offer steroid-free remission with disease control starting at 26 weeks is highly impactful for patients as well as providers.
I mean, I think this is a huge win.
Dr. Adam Brown
Absolutely. I totally agree this is a huge win for patients. Another thing that’s important for patients is their overall exposure to steroids. Let’s talk about what we saw at Week 52 as it relates to the cumulative steroid exposure. The RINVOQ patients had around 40% less cumulative exposure to steroids compared to the placebo arm. And most tapered off steroids by Week 26, or half the time compared to placebo.
To be exact, the median cumulative steroid exposure for the placebo patient was 2882mg versus 1615mg for the patients on RINVOQ.
Dr. Anisha Dua
That’s amazing. That’s a really substantial difference between the two arms in terms of cumulative steroid exposure. And this translates really to a major difference for patients, because we know that cumulative steroid exposure is really what drives a lot of these frustrating side effects for our patients. And to be able to offer them something that decreases that overall exposure rate is really, really important.
You know, after having seen so many patients of mine stuck on steroids for multiple years, it’s amazing to be able to offer a once-daily pill that can potentially get them off of steroids in half a year.
Dr. Adam Brown
Absolutely. I think patients would be excited about being on less steroids. Now, with any treatment, safety is a top priority. What about the safety of RINVOQ and JAK inhibitors, especially regarding some of the known risks associated with their use?
Dr. Anisha Dua
It’s important to remind our listeners that patients treated with RINVOQ are at an increased risk of serious infections that can lead to hospitalizations or death. Most of these infections occurred in patients who were concurrently taking immunosuppressants. A number of safety findings were observed in a study comparing another JAK inhibitor against TNF blockers in rheumatoid arthritis patients who were 50 and older and had one or more cardiovascular risk factors. A higher rate of all-cause mortality, thrombosis, lymphomas, and lung cancer was observed with the other JAK inhibitor.
Furthermore, the JAK inhibitor group demonstrated a higher rate of cardiovascular death, myocardial infarction, and stroke. There is increased risk for patients with a history of smoking.
Dr. Adam Brown
Those are all really important considerations. Thanks for sharing. Whenever I discuss RINVOQ with my patients, I make sure to have a conversation about both the potential risks and benefits of RINVOQ. It’s also important to note that malignancies also have been reported in patients with RINVOQ. Patients treated with JAK inhibitors for inflammatory conditions have also experienced deep venous thrombosis, pulmonary embolism, and arterial thrombosis.
Other serious adverse reactions associated with RINVOQ include hypersensitivity reactions, gastrointestinal perforations, laboratory abnormalities, and embryo-fetal toxicity.
Dr. Anisha Dua
So, in the SELECT-GCA trial, the average age of patients was 71 years old and 82% of patients were 65 and older. At baseline, these patients were on high doses of steroids.
Dr. Adam Brown
That sounds like a similar population to what I see in clinic. The first 52 weeks of the trial, the rates of serious infections, including opportunistic infections, were generally comparable between the RINVOQ and placebo arms. There’s a slightly higher rate of herpes zoster in the RINVOQ arm, so it’s important to counsel our patients on vaccination and monitor for infection.
Keep in mind that the placebo group in this study was an entire year of steroid taper, and thus experience side effects from that treatment as well. Something I found really important was there was no major adverse cardiovascular events reported in the RINVOQ arm, and 2.1 incidences per 100 patient-years reported in the placebo arm. The rates of malignancy events were similar between the groups, including non-melanoma skin cancer.
There was no events of lymphoma in either group. The overall rates of venous thromboembolism were comparable between the groups. There was also no events of gastrointestinal perforations or active tuberculosis seen in any arm of the trial.
Dr. Anisha Dua
That’s really important information to know, especially since we tend to be more cautious in our elderly patients who may have many comorbidities. And these are things that our patients will definitely be asking us about. It’s good to know that there were no major adverse cardiovascular events in the RINVOQ arm. And as you’re mentioning, herpes zoster is a known risk with JAK inhibitors.
What do we know about the patients in this trial? Were they vaccinated?
Dr. Adam Brown
Yeah. Good question. So, similar to the other RINVOQ clinical trials in rheumatology, most patients were not vaccinated. There was one person in the RINVOQ arm that had developed herpes zoster that had a prior vaccination. But no events of herpes zoster involved the CNS, liver or lungs.
Dr. Anisha Dua
And if I’m not mistaken, there were no new safety signals found in this trial, right?
Dr. Adam Brown
Correct. Yeah, there was no new safety signals identified for RINVOQ as a result of this clinical trial. Overall, the initial data suggests that the safety profile of RINVOQ for patients with GCA, an older population that may be at increased risk for complications is generally consistent with that safety profile in other approved indications. Dr. Anisha Dua, with a safety profile and demonstrated efficacy, what excites you about this option?
Dr. Anisha Dua
Well, it’s exciting to me that the FDA has approved RINVOQ as a first-line option for adult patients with GCA. We know it works. We know that there’s no new safety signals. We know that it decreases cumulative steroid exposure, and it’s an oral pill. So, it’s a great option for those who have trouble with injections. I have a lot of patients who travel a lot.
They’re unable to do infusions or carry injectables with them for long periods of time. So, it’s a nice option for them. And I think a lot of patients can potentially benefit from this steroid-sparing, effective agent.
Dr. Adam Brown
I couldn’t have said it better. It’s great for GCA patients and for providers to have RINVOQ as a treatment option. Thank you so much for taking the time today to share your insights into GCA, and for reviewing the SELECT-GCA trial data.
Dr. Anisha Dua
Absolutely. Thank you for having me. It’s been really fun to go over this exciting information and talk to you about it. And, also to learn a little bit about our commonalities and un- commonalities. Yeah. Yes. It has been a blast.
Dr. Adam Brown
The football tension in here.
Dr. Anisha Dua
Yeah, it’s building to a peak. We’re going to have to wrap it up, I guess.
Dr. Adam Brown
If you’ve enjoyed this episode and found it helpful, be sure to share it with your colleagues in rheumatology. Until next time, thanks for tuning in to The Breakout Rheum.
Narrator
INDICATIONS for RINVOQ (upadacitinib)
RINVOQ is indicated for the treatment of:
- Moderately to severely active rheumatoid arthritis (RA) in adults who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers.
- Active ankylosing spondylitis (AS) in adults who have had an inadequate response or intolerance to one or more TNF blockers.
- Active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation in adults who have had an inadequate response or intolerance to TNF blocker therapy.
- Giant cell arteritis (GCA) in adults.
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biologic disease-modifying antirheumatic drugs (bDMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine.
- Refractory, moderate to severe atopic dermatitis (AD) in adults and pediatric patients 12 years of age and older whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other immunosuppressants.
- Moderately to severely active ulcerative colitis (UC) in adults who have had an inadequate response or intolerance to one or more TNF blockers. If TNF blockers are clinically inadvisable, patients should have received at least one approved systemic therapy prior to use of RINVOQ.
- Moderately to severely active Crohn’s disease (CD) in adults who have had an inadequate response or intolerance to one or more TNF blockers. If TNF blockers are clinically inadvisable, patients should have received at least one approved systemic therapy prior to use of RINVOQ.
Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biological therapies for UC or CD, or with potent immunosuppressants such as azathioprine and cyclosporine.
RINVOQ/RINVOQ LQ is indicated for the treatment of:
- Active psoriatic arthritis (PsA) in adults and pediatric patients 2 years of age and older who have had an inadequate response or intolerance to one or more TNF blockers.
- Active polyarticular juvenile idiopathic arthritis (pJIA) in patients 2 years of age and older who have had an inadequate response or intolerance to one or more TNF blockers.
Limitations of Use: RINVOQ/RINVOQ LQ is not recommended for use in combination with other JAK inhibitors, bDMARDs, or with potent immunosuppressants such as azathioprine and cyclosporine.
IMPORTANT SAFETY INFORMATION FOR RINVOQ/RINVOQ LQ (upadacitinib)
SERIOUS INFECTIONS
Patients treated with RINVOQ* are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids. If a serious infection develops, interrupt RINVOQ until the infection is controlled. Reported infections include:
- Active tuberculosis (TB), which may present with pulmonary or extrapulmonary disease. Test patients for latent TB before RINVOQ use and during therapy. Consider treatment for latent TB infection prior to RINVOQ use.
- Invasive fungal infections, including cryptococcosis and pneumocystosis.
- Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
Carefully consider the risks and benefits of treatment with RINVOQ prior to initiating therapy in patients with chronic or recurrent infection. Monitor patients closely for the development of signs and symptoms of infection during and after treatment with RINVOQ, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy.
MORTALITY
In a large, randomized, postmarketing safety study comparing another Janus kinase (JAK) inhibitor with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients ≥50 years old with at least one cardiovascular (CV) risk factor, a higher rate of all-cause mortality, including sudden CV death, was observed with the JAK inhibitor. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ.
MALIGNANCIES
Lymphoma and other malignancies have been observed in patients treated with RINVOQ.
In a large, randomized, postmarketing safety study comparing another JAK inhibitor with TNF blockers in RA patients, a higher rate of malignancies (excluding non- melanoma skin cancer [NMSC]), lymphomas, and lung cancer (in current or past smokers) was observed with the JAK inhibitor. Patients who are current or past smokers are at additional increased risk.
With RINVOQ, consider the benefits and risks for the individual patient prior to initiating or continuing therapy, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers. NMSCs have been reported in patients treated with RINVOQ. Periodic skin examination is recommended for patients who are at increased risk for skin cancer. Advise patients to limit sunlight exposure by wearing protective clothing and using sunscreen.
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
In a large, randomized, postmarketing study comparing another JAK inhibitor with TNF blockers in RA patients ≥50 years old with at least one CV risk factor, a higher rate of MACE (defined as cardiovascular death, myocardial infarction, and stroke) was observed with the JAK inhibitor. Patients who are current or past smokers are at additional increased risk. Discontinue RINVOQ in patients that have experienced a myocardial infarction or stroke.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ, particularly in patients who are current or past smokers and patients with other CV risk factors. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
THROMBOSIS
Thromboses, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis, have occurred in patients treated for inflammatory conditions with JAK inhibitors, including RINVOQ. Many of these adverse events were serious and some resulted in death.
In a large, randomized, postmarketing study comparing another JAK inhibitor to TNF blockers in RA patients ≥50 years old with at least one CV risk factor, a higher rate of thrombosis was observed with the JAK inhibitor. Avoid RINVOQ in patients at risk. Patients with symptoms of thrombosis should discontinue RINVOQ and be promptly evaluated.
HYPERSENSITIVITY
RINVOQ is contraindicated in patients with known hypersensitivity to upadacitinib or any of its excipients. Serious hypersensitivity reactions, such as anaphylaxis and angioedema, were reported in patients receiving RINVOQ in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue RINVOQ and institute appropriate therapy.
GASTROINTESTINAL PERFORATIONS
Gastrointestinal (GI) perforations have been reported in clinical trials with RINVOQ. Monitor RINVOQ-treated patients who may be at risk for GI perforation (e.g., patients with a history of diverticulitis and patients taking NSAIDs or corticosteroids). Promptly evaluate patients presenting with new onset abdominal pain for early identification of GI perforation.
LABORATORY ABNORMALITIES
Neutropenia
Treatment with RINVOQ was associated with an increased incidence of neutropenia (absolute neutrophil count [ANC] <1000 cells/mm3). Treatment with RINVOQ is not recommended in patients with an ANC <1000 cells/mm3. Evaluate neutrophil counts at baseline and thereafter according to routine patient management.
Lymphopenia
Absolute lymphocyte counts (ALC) <500 cells/mm3 were reported in RINVOQ-treated patients. Treatment with RINVOQ is not recommended in patients with an ALC <500 cells/mm3. Evaluate at baseline and thereafter according to routine patient management.
Anemia
Decreases in hemoglobin levels to <8 g/dL were reported in RINVOQ-treated patients. Treatment should not be initiated or should be interrupted in patients with hemoglobin levels <8 g/dL. Evaluate at baseline and thereafter according to routine patient management.
Lipids
Treatment with RINVOQ was associated with increases in lipid parameters, including total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol. Manage patients according to clinical guidelines for the management of hyperlipidemia. Evaluate patients 12 weeks after initiation of treatment and thereafter according to the clinical guidelines for hyperlipidemia.
Liver enzyme elevations
Treatment with RINVOQ was associated with increased incidence of liver enzyme elevation compared to placebo. Evaluate at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. If increases in aspartate aminotransferase (AST) or alanine aminotransferase (ALT) are observed during routine patient management and drug-induced liver injury is suspected, RINVOQ should be interrupted until this diagnosis is excluded.
EMBRYO-FETAL TOXICITY
Based on findings in animal studies, RINVOQ may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with RINVOQ and for 4 weeks after the final dose. Verify pregnancy status of females of reproductive potential prior to starting treatment with RINVOQ.
VACCINATION
Avoid use of live vaccines during, or immediately prior to, RINVOQ therapy. Prior to initiating RINVOQ, patients should be brought up to date on all immunizations, including prophylactic varicella zoster or herpes zoster vaccinations, in agreement with current immunization guidelines.
MEDICATION RESIDUE IN STOOL
Reports of medication residue in stool or ostomy output have occurred in patients taking RINVOQ. Most reports described anatomic or functional GI conditions with shortened GI transit times. Instruct patients to contact their healthcare provider if medication residue is observed repeatedly. Monitor patients clinically and consider alternative treatment if there is an inadequate therapeutic response.
LACTATION
There are no data on the presence of RINVOQ in human milk, the effects on the breastfed infant, or the effects on milk production. Available data in animals have shown the excretion of RINVOQ in milk. Advise patients that breastfeeding is not recommended during treatment with RINVOQ and for 6 days after the last dose.
HEPATIC IMPAIRMENT
RINVOQ is not recommended for use in patients with severe hepatic impairment.
ADVERSE REACTIONS
The most common adverse reactions in RINVOQ clinical trials were upper respiratory tract infections, herpes zoster, herpes simplex, bronchitis, nausea, cough, pyrexia, acne, headache, peripheral edema, increased blood creatine phosphokinase, hypersensitivity, folliculitis, abdominal pain, increased weight, influenza, fatigue, neutropenia, myalgia, influenza-like illness, elevated liver enzymes, rash, and anemia.
Inform patients that retinal detachment has been reported in clinical trials with RINVOQ. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision while receiving RINVOQ.
Dosage Forms and Strengths: RINVOQ is available in 15 mg, 30 mg, and 45 mg extended-release tablets. RINVOQ LQ is available in a 1 mg/mL oral solution.
*Unless otherwise stated, “RINVOQ” in the IMPORTANT SAFETY INFORMATION refers to RINVOQ and RINVOQ LQ.
Please see accompanying full Prescribing Information for RINVOQ.