RINVOQ vs
HUMIRA® (adalimumab)
SELECT-SWITCH Study in RA
Explore head-to-head data for RINVOQ vs HUMIRA in moderate to severe rheumatoid arthritis patients who have had an inadequate response or intolerance to 1 TNFi.
US-MULT-250253
WARNING: Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis

*Clinical remission does not mean drug-free remission or complete absence of disease activity.
ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; CRP=C-reactive protein; DAS28-CRP=28-joint disease activity score using C-reactive protein.
aRINVOQ is on a preferred tier or otherwise has preferred status on the plan’s formulary.
bCoverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
INDICATION
RINVOQ is indicated for the treatment of adults with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers.
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biologic disease-modifying antirheumatic drugs (bDMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine.
*P<0.001. Analyses were not controlled for multiplicity. P-values obtained through nominal statistical testing.5
†In PsA: ~6.4 years maximum exposure (~3.6 years median) to RINVOQ 15 mg as of 08/2025. In AS: ~3.8 years maximum exposure (~1.8 years median) to RINVOQ 15 mg as of 08/2025. In nr-axSpA: ~2.3 years maximum exposure (~1.0 year median) to RINVOQ 15 mg as of 08/2025.7
‡RINVOQ is on a preferred tier or otherwise has preferred status on the plan's formulary.
§Coverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
AS=ankylosing spondylitis; bDMARD=biologic disease-modifying antirheumatic drug; csDMARD=conventional synthetic disease-modifying antirheumatic drug; IR=intolerance or inadequate response; max.=maximum; MTX=methotrexate; nr-axSpA=non-radiographic axial spondyloarthritis; PBO=placebo; PsA=psoriatic arthritis; RA=rheumatoid arthritis;
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
Primary Endpoint Results (Week 12 or 14)
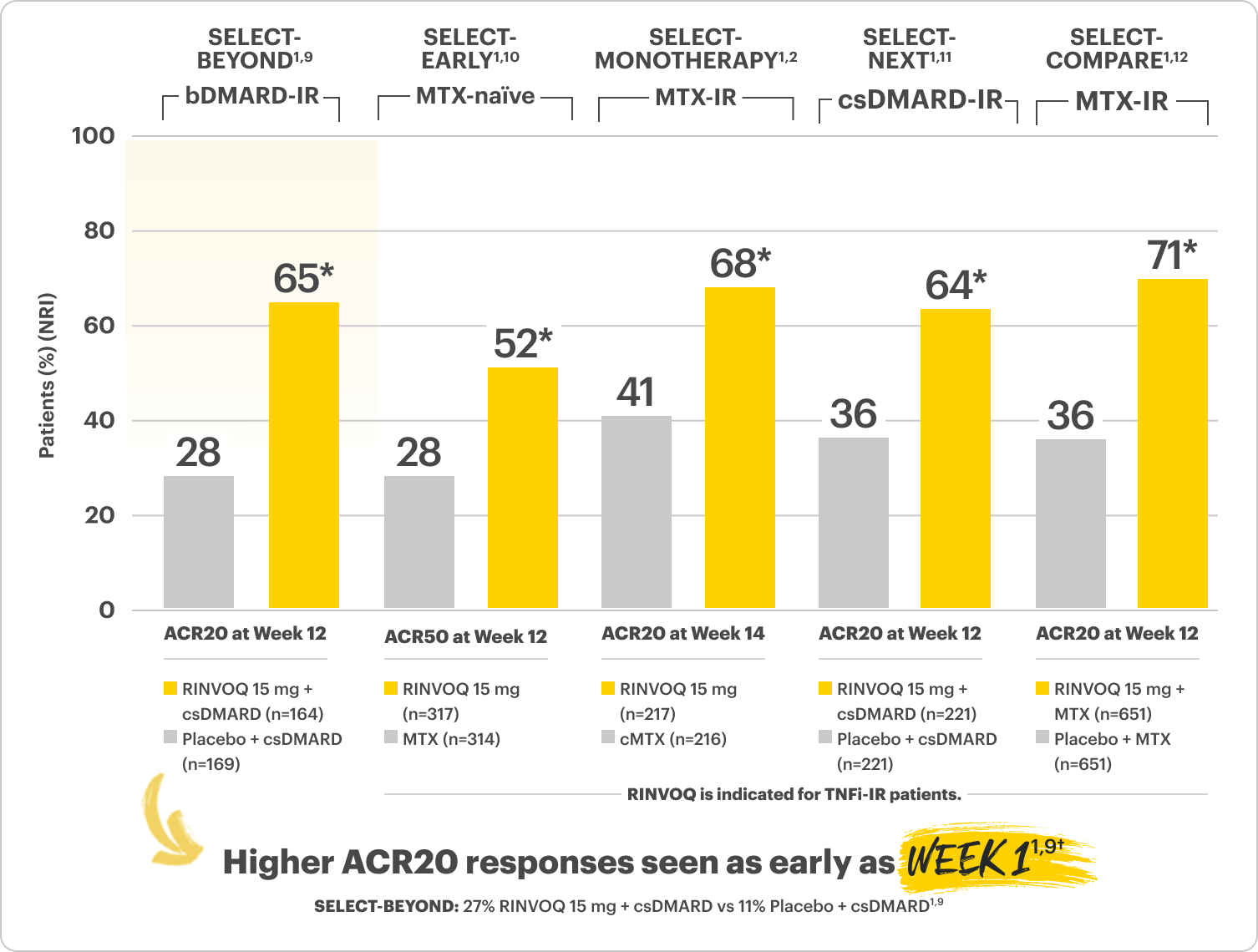
*P≤0.001 RINVOQ vs Placebo or MTX, †P=0.0001; Week 1 analyses were not controlled for multiplicity; P‑values obtained through nominal statistical testing.
SELECT-BEYOND (STUDY RA-V):1,9 24-week, randomized, double-blind, placebo-controlled study of 499 adult patients with moderate to severe RA who had an inadequate response or intolerance to bDMARDs.
SELECT-EARLY (STUDY RA-I):1,10 48-week, randomized, double-blind, active comparator-controlled study of 947 adult patients with moderate to severe RA who were MTX naïve.
SELECT-MONOTHERAPY (STUDY RA-II):1,2 14-week, randomized, double-blind, active comparator-controlled study of 648 adult patients with moderate to severe RA who had an inadequate response to MTX.
SELECT-NEXT (STUDY RA-III):1,11 12-week, randomized, double-blind, placebo-controlled study of 661 adult patients with moderate to severe RA who had an inadequate response to csDMARDs.
SELECT-COMPARE (STUDY RA-IV):1,12 48-week, randomized, double-blind, active comparator-controlled study of 1629 adult patients with moderate to severe RA who had an inadequate response to MTX.
ACR=American College of Rheumatology; bDMARD=biologic disease-modifying antirheumatic drug; cMTX=continued methotrexate; csDMARD=conventional synthetic disease-modifying antirheumatic drug; IR=intolerance or inadequate response; MTX=methotrexate; NRI=nonresponder imputation; RA=rheumatoid arthritis; TNFi=tumor necrosis factor inhibitor.
RINVOQ is indicated for the treatment of adults with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers.
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biologic disease-modifying antirheumatic drugs (bDMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine.
Patients treated with RINVOQ* are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids. If a serious infection develops, interrupt RINVOQ until the infection is controlled.
Reported infections include:
Carefully consider the risks and benefits of treatment with RINVOQ prior to initiating therapy in patients with chronic or recurrent infection. Monitor patients closely for the development of signs and symptoms of infection during and after treatment with RINVOQ, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy.
In a large, randomized, postmarketing safety study comparing another Janus kinase (JAK) inhibitor with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients ≥50 years old with at least one cardiovascular (CV) risk factor, a higher rate of all-cause mortality, including sudden CV death, was observed with the JAK inhibitor. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ.
Lymphoma and other malignancies have been observed in patients treated with RINVOQ.
In a large, randomized, postmarketing safety study comparing another JAK inhibitor with TNF blockers in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]), lymphomas, and lung cancer (in current or past smokers) was observed with the JAK inhibitor. Patients who are current or past smokers are at additional increased risk.
With RINVOQ, consider the benefits and risks for the individual patient prior to initiating or continuing therapy, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers. NMSCs have been reported in patients treated with RINVOQ. Periodic skin examination is recommended for patients who are at increased risk for skin cancer. Advise patients to limit sunlight exposure by wearing protective clothing and using sunscreen.
In a large, randomized, postmarketing study comparing another JAK inhibitor with TNF blockers in RA patients ≥50 years old with at least one CV risk factor, a higher rate of MACE (defined as cardiovascular death, myocardial infarction, and stroke) was observed with the JAK inhibitor. Patients who are current or past smokers are at additional increased risk. Discontinue RINVOQ in patients that have experienced a myocardial infarction or stroke.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ, particularly in patients who are current or past smokers and patients with other CV risk factors. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
Thromboses, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis, have occurred in patients treated for inflammatory conditions with JAK inhibitors, including RINVOQ. Many of these adverse events were serious and some resulted in death.
In a large, randomized, postmarketing study comparing another JAK inhibitor to TNF blockers in RA patients ≥50 years old with at least one CV risk factor, a higher rate of thrombosis was observed with the JAK inhibitor. Avoid RINVOQ in patients at risk. Patients with symptoms of thrombosis should discontinue RINVOQ and be promptly evaluated.
RINVOQ is contraindicated in patients with known hypersensitivity to upadacitinib or any of its excipients. Serious hypersensitivity reactions, such as anaphylaxis and angioedema, were reported in patients receiving RINVOQ in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue RINVOQ and institute appropriate therapy.
Gastrointestinal (GI) perforations have been reported in clinical trials with RINVOQ. Monitor RINVOQ-treated patients who may be at risk for GI perforation (e.g., patients with a history of diverticulitis and patients taking NSAIDs or corticosteroids). Promptly evaluate patients presenting with new onset abdominal pain for early identification of GI perforation.
Neutropenia
Treatment with RINVOQ was associated with an increased incidence of neutropenia (absolute neutrophil count [ANC] <1000 cells/mm3). Treatment with RINVOQ is not recommended in patients with an ANC <1000 cells/mm3. Evaluate neutrophil counts at baseline and thereafter according to routine patient management.
Lymphopenia
Absolute lymphocyte counts (ALC) <500 cells/mm3 were reported in RINVOQ-treated patients. Treatment with RINVOQ is not recommended in patients with an ALC <500 cells/mm3. Evaluate at baseline and thereafter according to routine patient management.
Anemia
Decreases in hemoglobin levels to <8 g/dL were reported in RINVOQ-treated patients. Treatment should not be initiated or should be interrupted in patients with hemoglobin levels <8 g/dL. Evaluate at baseline and thereafter according to routine patient management.
Lipids
Treatment with RINVOQ was associated with increases in lipid parameters, including total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol. Manage patients according to clinical guidelines for the management of hyperlipidemia. Evaluate patients 12 weeks after initiation of treatment and thereafter according to the clinical guidelines for hyperlipidemia.
Liver enzyme elevations
Treatment with RINVOQ was associated with increased incidence of liver enzyme elevation compared to placebo. Evaluate at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. If increases in aspartate aminotransferase (AST) or alanine aminotransferase (ALT) are observed during routine patient management and drug-induced liver injury is suspected, RINVOQ should be interrupted until this diagnosis is excluded.
Based on findings in animal studies, RINVOQ may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with RINVOQ and for 4 weeks after the final dose. Verify pregnancy status of females of reproductive potential prior to starting treatment with RINVOQ.
Avoid use of live vaccines during, or immediately prior to, RINVOQ therapy. Prior to initiating RINVOQ, patients should be brought up to date on all immunizations, including prophylactic varicella zoster or herpes zoster vaccinations, in agreement with current immunization guidelines.
Reports of medication residue in stool or ostomy output have occurred in patients taking RINVOQ. Most reports described anatomic or functional GI conditions with shortened GI transit times. Instruct patients to contact their healthcare provider if medication residue is observed repeatedly. Monitor patients clinically and consider alternative treatment if there is an inadequate therapeutic response.
There are no data on the presence of RINVOQ in human milk, the effects on the breastfed infant, or the effects on milk production. Available data in animals have shown the excretion of RINVOQ in milk. Advise patients that breastfeeding is not recommended during treatment with RINVOQ and for 6 days after the last dose.
RINVOQ is not recommended for use in patients with severe hepatic impairment.
The most common adverse reactions in RINVOQ clinical trials were upper respiratory tract infections, herpes zoster, herpes simplex, bronchitis, nausea, cough, pyrexia, acne, headache, peripheral edema, increased blood creatine phosphokinase, hypersensitivity, folliculitis, abdominal pain, increased weight, influenza, fatigue, neutropenia, myalgia, influenza-like illness, elevated liver enzymes, rash, and anemia.
Inform patients that retinal detachment has been reported in clinical trials with RINVOQ. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision while receiving RINVOQ.
Dosage Forms and Strengths: RINVOQ is available in 15 mg, 30 mg, and 45 mg extended-release tablets. RINVOQ LQ is available in a 1 mg/mL oral solution.
*Unless otherwise stated, “RINVOQ” in the IMPORTANT SAFETY INFORMATION refers to RINVOQ and RINVOQ LQ.
US-RNQ-250017
Please see full Prescribing Information.
REFERENCES:
SELECT-BEYOND
Adults with moderately to severely active RA who had an inadequate response or intolerance to bDMARDs1
RINVOQ is indicated for TNFi-IR patients1
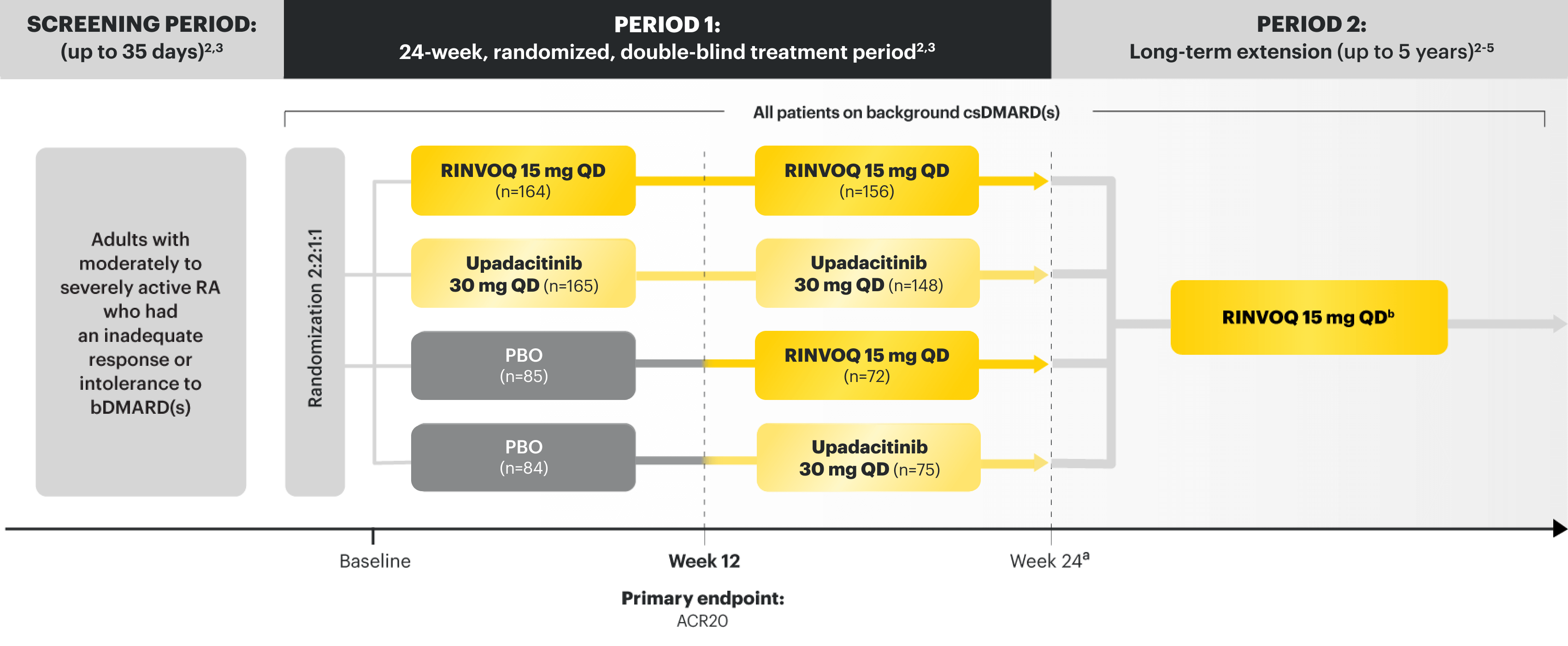
Upadacitinib 30 mg is not an approved dose.
aStarting at Week 24, initiation of or change in corticosteroids, NSAIDs, acetaminophen, and csDMARDs was permitted. Patients not achieving response criteria ≥20% improvement in SJC and TJC at 2 consecutive visits were removed from the study.4,5
bFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.8
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS6,7
At Week 12:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS7
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS6
| MEAN (SD) OR N (%) | PBO + csDMARDs n=169 | RINVOQ 15 mg + csDMARDs n=164 |
|---|---|---|
| Female, n (%) | 143 (85) | 137 (84) |
| Age (years), mean (SD) | 57.6 (11.4) | 56.3 (11.3) |
| Duration since RA diagnosis (years), mean (SD) | 14.5 (9.2) | 12.4 (9.4) |
| RF+ and/or ACPA+, n (%) | 128 (76) | 131 (80) |
| csDMARD use at baseline* | ||
| - MTX alone,† n (%) | 122 (73) | 118 (73) |
| - MTX plus other csDMARD,‡ n (%) | 17 (10) | 19 (12) |
| - MTX dose§ (mg), mean (SD) | 16.6 (4.7) | 17.3 (4.6) |
| - csDMARD other than MTX, n (%) | 29 (17) | 24 (15) |
| - Missing, n | 1 | 3 |
| Prior bDMARD exposure | ||
| - 1, n (%) | 83 (49) | 86 (52) |
| - 2, n (%) | 46 (27) | 40 (24) |
| - ≥3, n (%) | 40 (24) | 38 (23) |
| Inadequate response or intolerance to ≥1 anti-TNF drug | 152 (90) | 146 (89) |
| - Lack of efficacy with ≥1 bDMARD | 159 (94) | 146 (89) |
| - Lack of efficacy with ≥1 anti-IL-6 | 30 (18) | 27 (16) |
| Oral glucocorticoid use, n (%) | 74 (44) | 83 (51) |
| - Oral glucocorticoid dosell (mg), mean (SD) | 6.3 (2.4) | 5.7 (2.4) |
| TJC68, mean (SD) | 28.5 (15.3) | 27.8 (16.3) |
| SJC66, mean (SD) | 16.3 (9.6) | 17.0 (10.8) |
| PtGA (0-100 mm VAS), mean (SD) | 66.3 (22.7) | 67.2 (19.6) |
| PhGA (0-100 mm VAS), mean (SD) | 66.9 (16.9) | 68.7 (16.6) |
| Pain (0-100 mm VAS), mean (SD) | 68.9 (21.0) | 68.2 (19.8) |
| hsCRP (mg/L), mean (SD) | 16.3 (21.1) | 16.2 (18.6) |
| DAS28-CRP, mean (SD) | 5.8 (1.0) | 5.9 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.6) | 1.7 (0.6) |
| CDAI, mean (SD) | 41.0 (13.3) | 41.7 (13.3) |
| SDAI, mean (SD) | 42.6 (13.9) | 43.3 (13.8) |
*Oral or parenteral methotrexate (7.5-25 mg per week).
†Data available for 168 patients receiving placebo and 161 patients receiving RINVOQ 15 mg.
‡All combinations allowed except MTX and leflunomide.
§Mean MTX dose calculated only for patients receiving MTX.
‖Based on prednisone equivalent.
ACPA=anti‑citrullinated protein antibodies; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease‑modifying antirheumatic drug; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease‑modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high‑sensitivity C‑reactive protein; IL-6=interleukin 6; IR=intolerance or inadequate response; LDA=low disease activity; MTX=methotrexate; NSAID=nonsteroidal anti‑inflammatory drug; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36‑item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-EARLY
Adults with moderately to severely active RA who were MTX‑naïve1
RINVOQ is indicated for TNFi-IR patients1
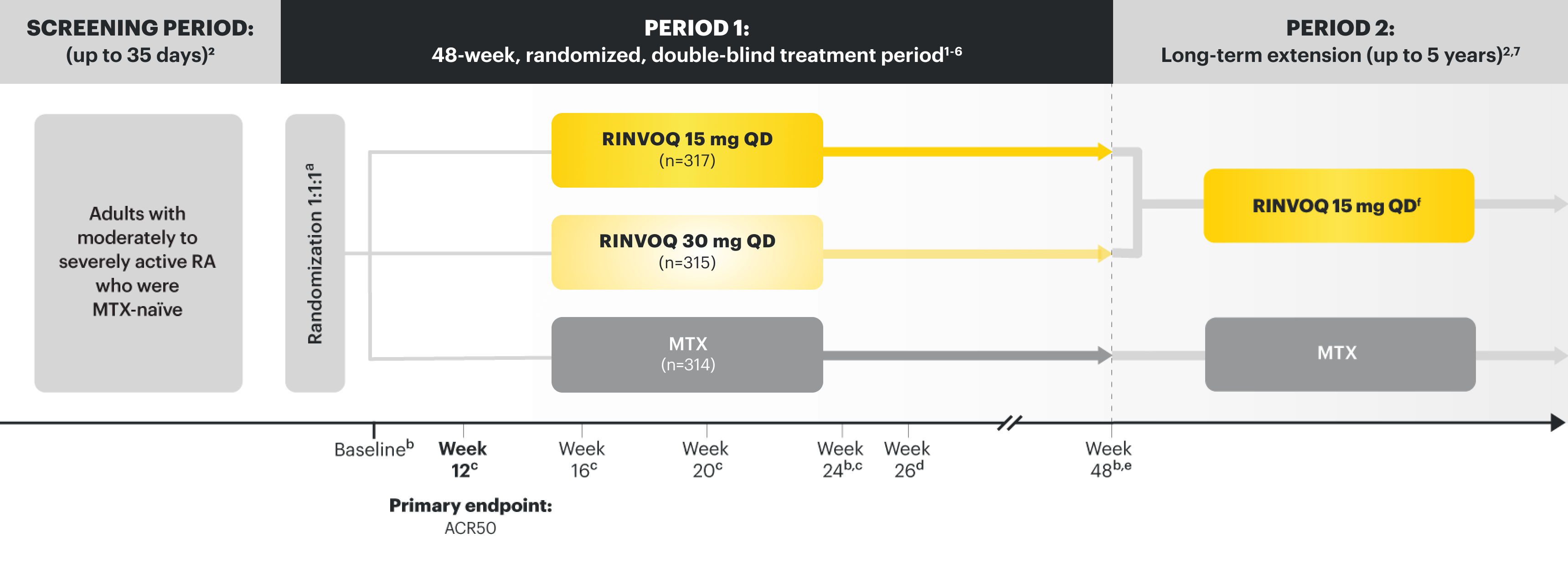
Upadacitinib 30 mg is not an approved dose.
aInitially 947 patients were randomized in the study, but 2 patients were never dosed.
bX-ray images of hands and feet obtained at these time points.2
cStarting at Week 12, patients with ≤20% improvement in TJC and SJC compared to baseline at 2 consecutive visits continued blinded therapy and optimized background RA medications (corticosteroids, NSAIDs, and/or low‑potency analgesics).3,4
dAt Week 26, patients with CDAI ≤2.8 continued their original study drug; background medications (NSAIDs, corticosteroids, and/or low‑potency analgesics, and csDMARDs) were optimized in patients with CDAI >2.8 but ≥20% improvement in TJC and SJC; among patients with CDAI >2.8 and <20% improvement in TJC and SJC, RINVOQ 15 mg or upadacitinib 30 mg were added by re‑randomization according to 1:1 ratio for those initially randomized to MTX, and MTX was added for those initially randomized to RINVOQ 15 mg or upadacitinib 30 mg.2,5
eStarting at Week 48, patients who did not achieve ≥20% improvement in both TJC and SJC at 2 consecutive visits were removed from the study. Initiation of or change in background RA medications (NSAIDs, corticosteroids, low-potency analgesics, and csDMARDs; not all patients received background MTX) is allowed at anytime during Period 2.6
fFollowing a protocol amendment, all patients in the long-term extension who were previously receiving UPA 30 mg received RINVOQ 15 mg.11
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS3,8
At Week 12:
At Week 24:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS2,3,9,10
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | MTX n=314 | RINVOQ 15 mg QD n=317 |
|---|---|---|
| Female, n (%) | 240 (76) | 241 (76) |
| Age (years), mean (SD) | 53.3 (12.9) | 51.9 (12.6) |
| Duration since RA diagnosis (years), mean (SD) | 2.6 (5.1) | 2.9 (5.4) |
| RF+ and/or ACPA+, n (%) | 255 (81) | 279 (88) |
| MTX exposure, n (%) | 19 (6) | 30 (9.5) |
| csDMARD exposure, n (%) | 79 (25) | 80 (25) |
| Oral glucocorticoid use, n (%) | 163 (52) | 147 (46) |
| - Dose (mg),* mean (SD) | 6.4 (2.4) | 6.4 (3.1) |
| TJC68, mean (SD) | 26.4 (16.2) | 25.4 (14.4) |
| SJC66, mean (SD) | 16.9 (10.6) | 16.9 (10.4) |
| PtGA (0-100 mm VAS), mean (SD) | 65.8 (21.5) | 66.6 (22.0) |
| PhGA (0-100 mm VAS), mean (SD) | 68.7 (16.5) | 67.1 (17.0) |
| Pain (0-100 mm VAS), mean (SD) | 65.7 (21.5) | 68.4 (20.6) |
| hsCRP (mg/L), mean (SD) | 21.2 (22.1) | 23.0 (27.4) |
| DAS28-CRP, mean (SD) | 5.9 (1.0) | 5.9 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.7) | 1.6 (0.7) |
| CDAI, mean (SD) | 40.5 (13.3) | 40.4 (13.3) |
| SDAI, mean (SD) | 42.6 (14.0) | 42.7 (13.9) |
| mTSS, mean (SD) | 13.3 (30.6) | 18.1 (38.2) |
| - Erosion Score, mean (SD) | 6.1 (15.5) | 8.6 (19.3) |
| - Joint Space Narrowing Score, mean (SD) | 7.2 (16.2) | 9.6 (20.1) |
*Prednisone equivalent dose in patients receiving oral glucocorticoids at baseline.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C-reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; HAQ-DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C-reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; NSAID=nonsteroidal anti-inflammatory drug; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF-36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-MONOTHERAPY
Adults with moderately to severely active RA who had an inadequate response to MTX1
RINVOQ is indicated for TNFi-IR patients1
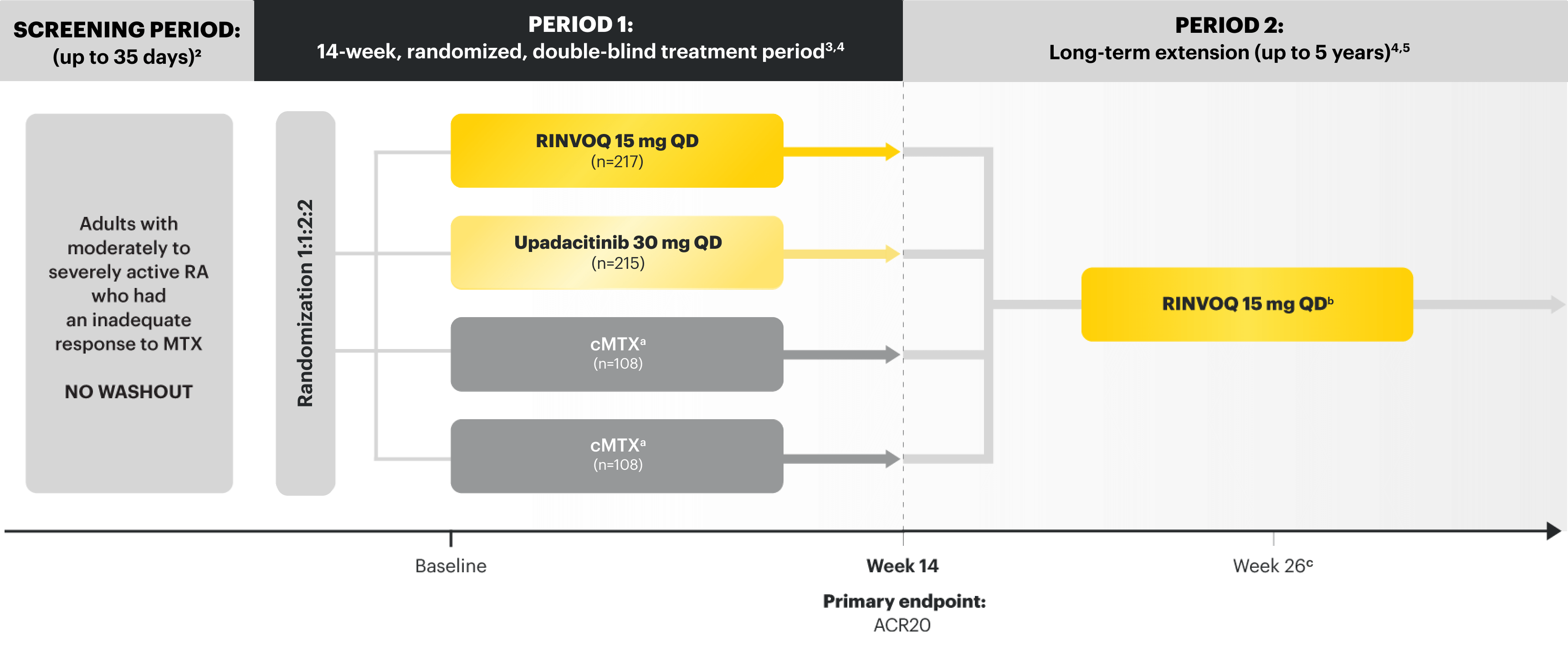
Upadacitinib 30 mg is not an approved dose.
aPatients on cMTX were randomized to receive either RINVOQ 15 mg or upadacitinib 30 mg at Week 14.3
bStarting at Week 26, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDs, acetaminophen, or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.5
cFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.4
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS3,6
At Week 14:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS3,6
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | cMTX n=216 | RINVOQ 15 mg QD n=217 |
|---|---|---|
| Female, n (%) | 179 (83) | 174 (80) |
| Age (years), mean (SD) | 55.3 (11.1) | 54.5 (12.2) |
| Duration since RA diagnosis (years), mean (SD) | 5.8 (6.6) | 7.5 (8.9) |
| RF+ and/or ACPA+, n (%) | 169 (78) | 172 (79) |
| Prior MTX dose* (mg/week), mean (SD) | 16.7 (4.4) | 16.8 (4.2) |
| Prior MTX duration (years), mean (SD) | 3.3 (3.9) | 3.8 (4.8) |
| Oral glucocorticoid use, n (%) | 115 (53) | 114 (53) |
| - Oral glucocorticoid dose† (mg), mean (SD) | 6.2 (2.6) | 6.1 (2.5) |
| TJC68, mean (SD) | 25.2 (16.0) | 24.5 (15.1) |
| SJC66, mean (SD) | 16.9 (11.5) | 16.4 (10.9) |
| PtGA (0-100 mm VAS), mean (SD) | 59.6 (21.8) | 62.2 (22.3) |
| PhGA (0-100 mm VAS), mean (SD) | 62.1 (17.5) | 65.7 (18.5) |
| Pain (0-100 mm VAS), mean (SD) | 62.5 (21.3) | 62.3 (22.5) |
| hsCRP (mg/L), mean (SD) | 14.5 (17.3) | 14.0 (16.5) |
| DAS28-CRP, mean (SD) | 5.6 (1.0) | 5.6 (0.9) |
| HAQ-DI, mean (SD) | 1.5 (0.7) | 1.5 (0.7) |
| CDAI, mean (SD) | 37.8 (14.4) | 38.0 (13.1) |
| SDAI, mean (SD) | 39.2 (14.6) | 39.4 (13.4) |
*Prior to receiving study drug. In the control arm, patients continued prior MTX dose as blinded study drug.3
†Prednisone equivalent.
ACPA=anti‑citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=Clinical Disease Activity Index; cMTX=continuous methotrexate; csDMARD=conventional synthetic disease‑modifying antirheumatic drug; CR=clinical remission; CRP=C‑reactive protein; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C‑reactive protein; IR=intolerance or inadequate response; LDA=low disease activity; MTX=methotrexate; NSAID=nonsteroidal anti‑inflammatory drug; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-NEXT
Adults with moderately to severely active RA who had an inadequate response to csDMARD(s)1
RINVOQ is indicated for TNFi-IR patients1
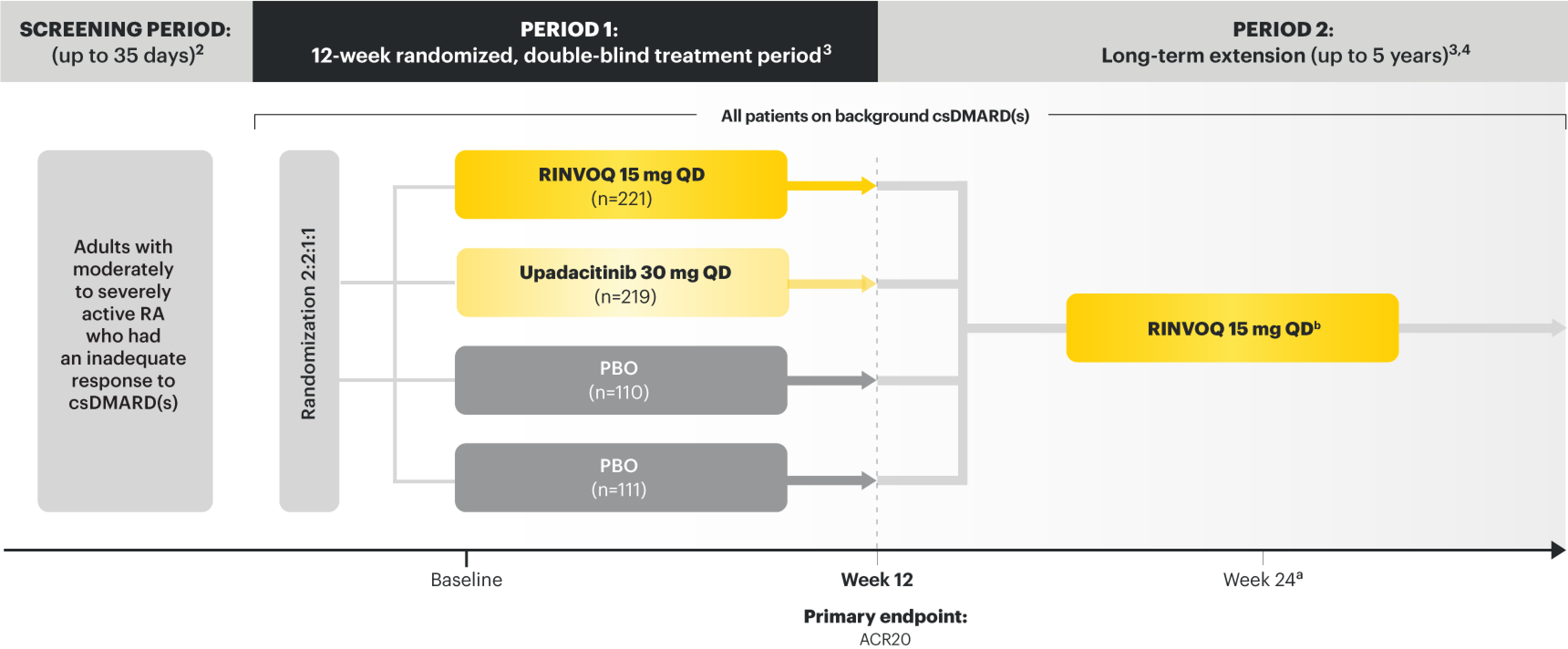
Upadacitinib 30 mg is not an approved dose.
aStarting at Week 24, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDs, acetaminophen, or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.4
bFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.7
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS5,6
At Week 12:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS6
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | PBO + csDMARDs n=221 | RINVOQ 15 mg QD + csDMARDs n=221 |
|---|---|---|
| Female, n (%) | 166 (75) | 182 (82) |
| Age (years), mean (SD) | 56.0 (12.2) | 55.3 (11.5) |
| Duration since RA diagnosis (years), mean (SD) | 7.2 (7.5) | 7.3 (7.9) |
| RF+ and/or ACPA+, n (%) | 181 (82) | 184 (83) |
| csDMARD use at baseline | ||
| - MTX alone, n (%) | 141 (64) | 122 (55) |
| - MTX plus other csDMARD, n (%) | 49 (22) | 47 (21) |
| - csDMARD other than MTX, n (%) | 30 (14) | 51 (23) |
| - Missing, n (%) | 1 (<1) | 1 (<1) |
| Prior bDMARD exposure, n (%) | 29 (13) | 27 (12) |
| Oral glucocorticoid use, n (%) | 106 (48) | 96 (43) |
| - Oral glucocorticoid dose* (mg), mean (SD) | 6.3 (2.6) | 6.0 (2.4) |
| TJC68, mean (SD) | 24.7 (15.0) | 25.2 (13.8) |
| SJC66, mean (SD) | 15.4 (9.2) | 16.0 (10.0) |
| PtGA (0-100 mm VAS), mean (SD) | 60.3 (20.5) | 63.1 (21.9) |
| PhGA (0-100 mm VAS), mean (SD) | 64.4 (17.7) | 64.3 (16.2) |
| Pain (0-100 mm VAS), mean (SD) | 61.5 (20.8) | 64.1 (19.5) |
| hsCRP (mg/L), mean (SD) | 12.6 (14.0) | 16.6 (19.2) |
| DAS28-CRP, mean (SD) | 5.6 (0.8) | 5.7 (1.0) |
| HAQ-DI, mean (SD) | 1.4 (0.6) | 1.5 (0.6) |
| CDAI, mean (SD) | 37.8 (11.8) | 38.3 (11.9) |
| SDAI, mean (SD) | 39.0 (11.9) | 39.9 (12.5) |
*Based on prednisone equivalent.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease-modifying antirheumatic drug; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C-reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; HAQ-DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C-reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; MTX=methotrexate; NSAIDs=nonsteroidal anti-inflammatory drugs; PBO=placebo; PhGA=physician's global assessment of disease activity; PtGA=patient's global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-COMPARE
Adults with moderately to severely active RA who had an inadequate response to MTX1
RINVOQ is indicated for TNFi-IR patients1
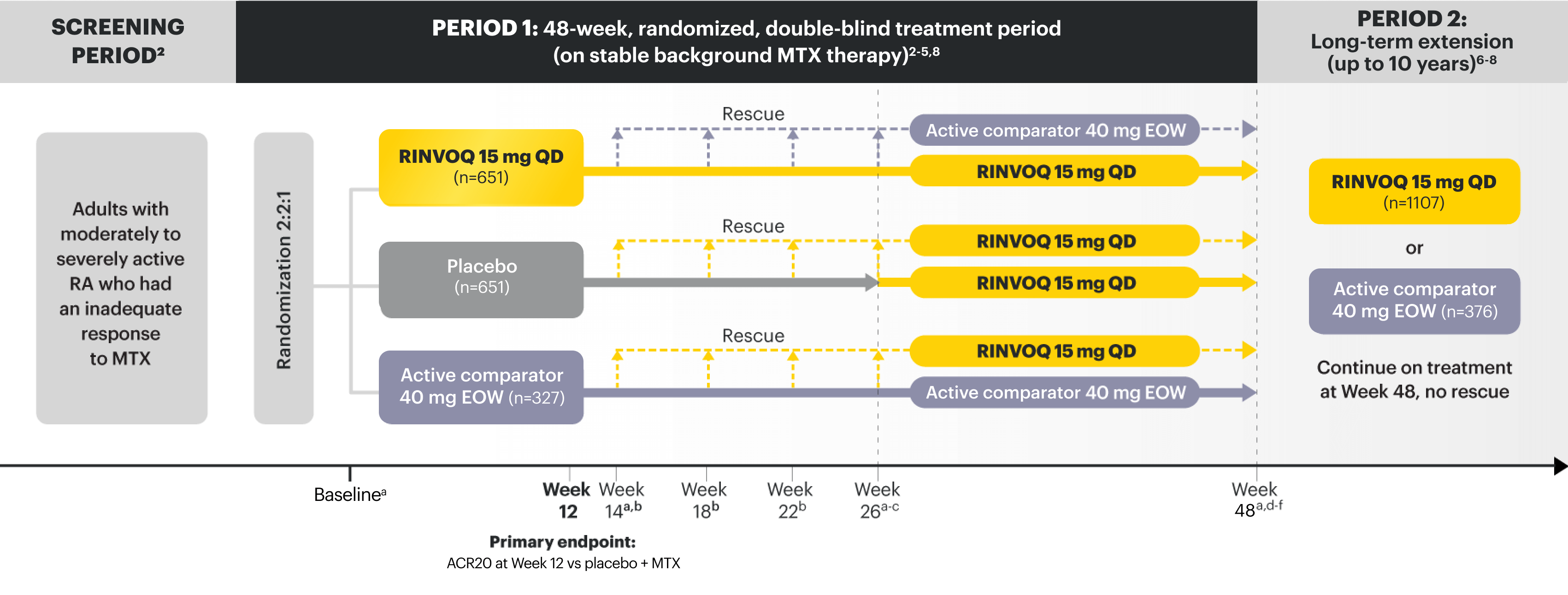
aX-ray imaging was performed at these time points; Week 14 for nonresponder patients, who were rescued.2,3
bRescue criteria: At Weeks 14, 18, and 22, if <20% improvement in TJC and SJC vs baseline; at Week 26, all remaining PBO patients were switched to RINVOQ, and patients receiving RINVOQ or active comparator were switched to active comparator or RINVOQ, respectively, if CDAI >10.2
cStarting at Week 26, initiation or change in background RA medication(s), including corticosteroids, NSAIDs, or acetaminophen was permitted.4
dStarting at Week 48, patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.5
eAt Week 48, initiation or change in csDMARDs was allowed; however, not all patients received background MTX.5
fPatients continued treatment with UPA or active comparator in a blinded manner until the last patient completed the Week 48 visit and received open-label treatment thereafter.8
PRIMARY ENDPOINT1
SELECT RANKED SECONDARY ENDPOINTS2
At Week 12 vs placebo + MTX:
At Week 12 vs active comparator + MTX:
At Week 26 vs placebo + MTX:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS3,9,10
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
SELECT-COMPARE was not designed to evaluate the efficacy of active comparator + MTX vs placebo + MTX. No conclusions regarding this comparison can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | PBO + MTX n=651 | RINVOQ 15 mg QD + MTX n=651 |
|---|---|---|
| Female, n (%) | 512 (79) | 521 (80) |
| Age (years), mean (SD) | 54 (12) | 54 (12) |
| Duration since RA diagnosis (years), mean (SD) | 8 (8) | 8 (8) |
| RF+ and/or anti-CCP+, n (%) | 571 (88) | 566 (87) |
| MTX dose (mg/week), mean (SD) | 16.8 (3.8) | 17.0 (4.2) |
| Prior bDMARD use, n (%) | 63 (10) | 54 (8) |
| Oral glucocorticoid use, n (%) | 392 (60) | 388 (60) |
| - Dose (mg),* mean (SD) | 6.3 (2.4) | 6.2 (2.3) |
| TJC68, mean (SD) | 26 (14) | 26 (15) |
| SJC66, mean (SD) | 16 (9) | 17 (10) |
| PtGA (0-100 mm VAS), mean (SD) | 64 (21) | 64 (22) |
| PhGA (0-100 mm VAS), mean (SD) | 66 (18) | 66 (17) |
| Pain (0-100 mm VAS), mean (SD) | 65 (21) | 66 (21) |
| hsCRP (mg/L), mean (SD) | 18 (22) | 18 (22) |
| DAS28-CRP, mean (SD) | 5.8 (0.9) | 5.8 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.6) | 1.6 (0.6) |
| CDAI, mean (SD) | 40 (13) | 40 (13) |
| SDAI, mean (SD) | 41.8 (13.3) | 41.5 (13.6) |
| mTSS, mean (SD) | 36 (52) | 34 (50) |
| - JE score, mean (SD) | 17 (27) | 17 (26) |
| - JSN score, mean (SD) | 19 (26) | 18 (25) |
*Based on prednisone equivalent.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease‑modifying antirheumatic drug; CCP=cyclic citrullinated peptide; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; EOW=every other week; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C‑reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; NSAID=nonsteroidal anti-inflammatory drug; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once per day; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
US-RNQ-230377
SELECT-BEYOND
Adults with moderately to severely active RA who had an inadequate response or intolerance to bDMARDs1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aStarting at Week 24, initiation of or change in corticosteroids, NSAIDs, acetaminophen, and csDMARDs was permitted. Patients not achieving response criteria ≥20% improvement in SJC and TJC at 2 consecutive visits were removed from the study.4,5
bFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.8
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS6,7
At Week 12:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS7
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS6
| MEAN (SD) OR N (%) | PBO + csDMARDs n=169 | RINVOQ 15 mg + csDMARDs n=164 |
|---|---|---|
| Female, n (%) | 143 (85) | 137 (84) |
| Age (years), mean (SD) | 57.6 (11.4) | 56.3 (11.3) |
| Duration since RA diagnosis (years), mean (SD) | 14.5 (9.2) | 12.4 (9.4) |
| RF+ and/or ACPA+, n (%) | 128 (76) | 131 (80) |
| csDMARD use at baseline* | ||
| - MTX alone,† n (%) | 122 (73) | 118 (73) |
| - MTX plus other csDMARD,‡ n (%) | 17 (10) | 19 (12) |
| - MTX dose§ (mg), mean (SD) | 16.6 (4.7) | 17.3 (4.6) |
| - csDMARD other than MTX, n (%) | 29 (17) | 24 (15) |
| - Missing, n | 1 | 3 |
| Prior bDMARD exposure | ||
| - 1, n (%) | 83 (49) | 86 (52) |
| - 2, n (%) | 46 (27) | 40 (24) |
| - ≥3, n (%) | 40 (24) | 38 (23) |
| Inadequate response or intolerance to ≥1 anti-TNF drug | 152 (90) | 146 (89) |
| - Lack of efficacy with ≥1 bDMARD | 159 (94) | 146 (89) |
| - Lack of efficacy with ≥1 anti-IL-6 | 30 (18) | 27 (16) |
| Oral glucocorticoid use, n (%) | 74 (44) | 83 (51) |
| - Oral glucocorticoid dosell (mg), mean (SD) | 6.3 (2.4) | 5.7 (2.4) |
| TJC68, mean (SD) | 28.5 (15.3) | 27.8 (16.3) |
| SJC66, mean (SD) | 16.3 (9.6) | 17.0 (10.8) |
| PtGA (0-100 mm VAS), mean (SD) | 66.3 (22.7) | 67.2 (19.6) |
| PhGA (0-100 mm VAS), mean (SD) | 66.9 (16.9) | 68.7 (16.6) |
| Pain (0-100 mm VAS), mean (SD) | 68.9 (21.0) | 68.2 (19.8) |
| hsCRP (mg/L), mean (SD) | 16.3 (21.1) | 16.2 (18.6) |
| DAS28-CRP, mean (SD) | 5.8 (1.0) | 5.9 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.6) | 1.7 (0.6) |
| CDAI, mean (SD) | 41.0 (13.3) | 41.7 (13.3) |
| SDAI, mean (SD) | 42.6 (13.9) | 43.3 (13.8) |
*Oral or parenteral methotrexate (7.5-25 mg per week).
†Data available for 168 patients receiving placebo and 161 patients receiving RINVOQ 15 mg.
‡All combinations allowed except MTX and leflunomide.
§Mean MTX dose calculated only for patients receiving MTX.
‖Based on prednisone equivalent.
ACPA=anti‑citrullinated protein antibodies; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease‑modifying antirheumatic drug; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease‑modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high‑sensitivity C‑reactive protein; IL-6=interleukin 6; IR=intolerance or inadequate response; LDA=low disease activity; MTX=methotrexate; NSAID=nonsteroidal anti‑inflammatory drug; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36‑item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-EARLY
Adults with moderately to severely active RA who were MTX‑naïve1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aInitially 947 patients were randomized in the study, but 2 patients were never dosed.
bX-ray images of hands and feet obtained at these time points.2
cStarting at Week 12, patients with ≤20% improvement in TJC and SJC compared to baseline at 2 consecutive visits continued blinded therapy and optimized background RA medications (corticosteroids, NSAIDs, and/or low‑potency analgesics).3,4
dAt Week 26, patients with CDAI ≤2.8 continued their original study drug; background medications (NSAIDs, corticosteroids, and/or low‑potency analgesics, and csDMARDs) were optimized in patients with CDAI >2.8 but ≥20% improvement in TJC and SJC; among patients with CDAI >2.8 and <20% improvement in TJC and SJC, RINVOQ 15 mg or upadacitinib 30 mg were added by re‑randomization according to 1:1 ratio for those initially randomized to MTX, and MTX was added for those initially randomized to RINVOQ 15 mg or upadacitinib 30 mg.2,5
eStarting at Week 48, patients who did not achieve ≥20% improvement in both TJC and SJC at 2 consecutive visits were removed from the study. Initiation of or change in background RA medications (NSAIDs, corticosteroids, low-potency analgesics, and csDMARDs; not all patients received background MTX) is allowed at anytime during Period 2.6
fFollowing a protocol amendment, all patients in the long-term extension who were previously receiving UPA 30 mg received RINVOQ 15 mg.11
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS3,8
At Week 12:
At Week 24:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS2,3,9,10
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | MTX n=314 | RINVOQ 15 mg QD n=317 |
|---|---|---|
| Female, n (%) | 240 (76) | 241 (76) |
| Age (years), mean (SD) | 53.3 (12.9) | 51.9 (12.6) |
| Duration since RA diagnosis (years), mean (SD) | 2.6 (5.1) | 2.9 (5.4) |
| RF+ and/or ACPA+, n (%) | 255 (81) | 279 (88) |
| MTX exposure, n (%) | 19 (6) | 30 (9.5) |
| csDMARD exposure, n (%) | 79 (25) | 80 (25) |
| Oral glucocorticoid use, n (%) | 163 (52) | 147 (46) |
| - Dose (mg),* mean (SD) | 6.4 (2.4) | 6.4 (3.1) |
| TJC68, mean (SD) | 26.4 (16.2) | 25.4 (14.4) |
| SJC66, mean (SD) | 16.9 (10.6) | 16.9 (10.4) |
| PtGA (0-100 mm VAS), mean (SD) | 65.8 (21.5) | 66.6 (22.0) |
| PhGA (0-100 mm VAS), mean (SD) | 68.7 (16.5) | 67.1 (17.0) |
| Pain (0-100 mm VAS), mean (SD) | 65.7 (21.5) | 68.4 (20.6) |
| hsCRP (mg/L), mean (SD) | 21.2 (22.1) | 23.0 (27.4) |
| DAS28-CRP, mean (SD) | 5.9 (1.0) | 5.9 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.7) | 1.6 (0.7) |
| CDAI, mean (SD) | 40.5 (13.3) | 40.4 (13.3) |
| SDAI, mean (SD) | 42.6 (14.0) | 42.7 (13.9) |
| mTSS, mean (SD) | 13.3 (30.6) | 18.1 (38.2) |
| - Erosion Score, mean (SD) | 6.1 (15.5) | 8.6 (19.3) |
| - Joint Space Narrowing Score, mean (SD) | 7.2 (16.2) | 9.6 (20.1) |
*Prednisone equivalent dose in patients receiving oral glucocorticoids at baseline.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C-reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; HAQ-DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C-reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; NSAID=nonsteroidal anti-inflammatory drug; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF-36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-MONOTHERAPY
Adults with moderately to severely active RA who had an inadequate response to MTX1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aPatients on cMTX were randomized to receive either RINVOQ 15 mg or upadacitinib 30 mg at Week 14.3
bStarting at Week 26, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDs, acetaminophen, or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.5
cFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.4
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS3,6
At Week 14:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS3,6
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | cMTX n=216 | RINVOQ 15 mg QD n=217 |
|---|---|---|
| Female, n (%) | 179 (83) | 174 (80) |
| Age (years), mean (SD) | 55.3 (11.1) | 54.5 (12.2) |
| Duration since RA diagnosis (years), mean (SD) | 5.8 (6.6) | 7.5 (8.9) |
| RF+ and/or ACPA+, n (%) | 169 (78) | 172 (79) |
| Prior MTX dose* (mg/week), mean (SD) | 16.7 (4.4) | 16.8 (4.2) |
| Prior MTX duration (years), mean (SD) | 3.3 (3.9) | 3.8 (4.8) |
| Oral glucocorticoid use, n (%) | 115 (53) | 114 (53) |
| - Oral glucocorticoid dose† (mg), mean (SD) | 6.2 (2.6) | 6.1 (2.5) |
| TJC68, mean (SD) | 25.2 (16.0) | 24.5 (15.1) |
| SJC66, mean (SD) | 16.9 (11.5) | 16.4 (10.9) |
| PtGA (0-100 mm VAS), mean (SD) | 59.6 (21.8) | 62.2 (22.3) |
| PhGA (0-100 mm VAS), mean (SD) | 62.1 (17.5) | 65.7 (18.5) |
| Pain (0-100 mm VAS), mean (SD) | 62.5 (21.3) | 62.3 (22.5) |
| hsCRP (mg/L), mean (SD) | 14.5 (17.3) | 14.0 (16.5) |
| DAS28-CRP, mean (SD) | 5.6 (1.0) | 5.6 (0.9) |
| HAQ-DI, mean (SD) | 1.5 (0.7) | 1.5 (0.7) |
| CDAI, mean (SD) | 37.8 (14.4) | 38.0 (13.1) |
| SDAI, mean (SD) | 39.2 (14.6) | 39.4 (13.4) |
*Prior to receiving study drug. In the control arm, patients continued prior MTX dose as blinded study drug.3
†Prednisone equivalent.
ACPA=anti‑citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=Clinical Disease Activity Index; cMTX=continuous methotrexate; csDMARD=conventional synthetic disease‑modifying antirheumatic drug; CR=clinical remission; CRP=C‑reactive protein; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C‑reactive protein; IR=intolerance or inadequate response; LDA=low disease activity; MTX=methotrexate; NSAID=nonsteroidal anti‑inflammatory drug; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-NEXT
Adults with moderately to severely active RA who had an inadequate response to csDMARD(s)1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aStarting at Week 24, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDs, acetaminophen, or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.4
bFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.7
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS5,6
At Week 12:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS6
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | PBO + csDMARDs n=221 | RINVOQ 15 mg QD + csDMARDs n=221 |
|---|---|---|
| Female, n (%) | 166 (75) | 182 (82) |
| Age (years), mean (SD) | 56.0 (12.2) | 55.3 (11.5) |
| Duration since RA diagnosis (years), mean (SD) | 7.2 (7.5) | 7.3 (7.9) |
| RF+ and/or ACPA+, n (%) | 181 (82) | 184 (83) |
| csDMARD use at baseline | ||
| - MTX alone, n (%) | 141 (64) | 122 (55) |
| - MTX plus other csDMARD, n (%) | 49 (22) | 47 (21) |
| - csDMARD other than MTX, n (%) | 30 (14) | 51 (23) |
| - Missing, n (%) | 1 (<1) | 1 (<1) |
| Prior bDMARD exposure, n (%) | 29 (13) | 27 (12) |
| Oral glucocorticoid use, n (%) | 106 (48) | 96 (43) |
| - Oral glucocorticoid dose* (mg), mean (SD) | 6.3 (2.6) | 6.0 (2.4) |
| TJC68, mean (SD) | 24.7 (15.0) | 25.2 (13.8) |
| SJC66, mean (SD) | 15.4 (9.2) | 16.0 (10.0) |
| PtGA (0-100 mm VAS), mean (SD) | 60.3 (20.5) | 63.1 (21.9) |
| PhGA (0-100 mm VAS), mean (SD) | 64.4 (17.7) | 64.3 (16.2) |
| Pain (0-100 mm VAS), mean (SD) | 61.5 (20.8) | 64.1 (19.5) |
| hsCRP (mg/L), mean (SD) | 12.6 (14.0) | 16.6 (19.2) |
| DAS28-CRP, mean (SD) | 5.6 (0.8) | 5.7 (1.0) |
| HAQ-DI, mean (SD) | 1.4 (0.6) | 1.5 (0.6) |
| CDAI, mean (SD) | 37.8 (11.8) | 38.3 (11.9) |
| SDAI, mean (SD) | 39.0 (11.9) | 39.9 (12.5) |
*Based on prednisone equivalent.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease-modifying antirheumatic drug; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C-reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; HAQ-DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C-reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; MTX=methotrexate; NSAIDs=nonsteroidal anti-inflammatory drugs; PBO=placebo; PhGA=physician's global assessment of disease activity; PtGA=patient's global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-COMPARE
Adults with moderately to severely active RA who had an inadequate response to MTX1
RINVOQ is indicated for TNFi-IR patients1

aX-ray imaging was performed at these time points; Week 14 for nonresponder patients, who were rescued.2,3
bRescue criteria: At Weeks 14, 18, and 22, if <20% improvement in TJC and SJC vs baseline; at Week 26, all remaining PBO patients were switched to RINVOQ, and patients receiving RINVOQ or active comparator were switched to active comparator or RINVOQ, respectively, if CDAI >10.2
cStarting at Week 26, initiation or change in background RA medication(s), including corticosteroids, NSAIDs, or acetaminophen was permitted.4
dStarting at Week 48, patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.5
eAt Week 48, initiation or change in csDMARDs was allowed; however, not all patients received background MTX.5
fPatients continued treatment with UPA or active comparator in a blinded manner until the last patient completed the Week 48 visit and received open-label treatment thereafter.8
PRIMARY ENDPOINT1
SELECT RANKED SECONDARY ENDPOINTS2
At Week 12 vs placebo + MTX:
At Week 12 vs active comparator + MTX:
At Week 26 vs placebo + MTX:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS3,9,10
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
SELECT-COMPARE was not designed to evaluate the efficacy of active comparator + MTX vs placebo + MTX. No conclusions regarding this comparison can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | PBO + MTX n=651 | RINVOQ 15 mg QD + MTX n=651 |
|---|---|---|
| Female, n (%) | 512 (79) | 521 (80) |
| Age (years), mean (SD) | 54 (12) | 54 (12) |
| Duration since RA diagnosis (years), mean (SD) | 8 (8) | 8 (8) |
| RF+ and/or anti-CCP+, n (%) | 571 (88) | 566 (87) |
| MTX dose (mg/week), mean (SD) | 16.8 (3.8) | 17.0 (4.2) |
| Prior bDMARD use, n (%) | 63 (10) | 54 (8) |
| Oral glucocorticoid use, n (%) | 392 (60) | 388 (60) |
| - Dose (mg),* mean (SD) | 6.3 (2.4) | 6.2 (2.3) |
| TJC68, mean (SD) | 26 (14) | 26 (15) |
| SJC66, mean (SD) | 16 (9) | 17 (10) |
| PtGA (0-100 mm VAS), mean (SD) | 64 (21) | 64 (22) |
| PhGA (0-100 mm VAS), mean (SD) | 66 (18) | 66 (17) |
| Pain (0-100 mm VAS), mean (SD) | 65 (21) | 66 (21) |
| hsCRP (mg/L), mean (SD) | 18 (22) | 18 (22) |
| DAS28-CRP, mean (SD) | 5.8 (0.9) | 5.8 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.6) | 1.6 (0.6) |
| CDAI, mean (SD) | 40 (13) | 40 (13) |
| SDAI, mean (SD) | 41.8 (13.3) | 41.5 (13.6) |
| mTSS, mean (SD) | 36 (52) | 34 (50) |
| - JE score, mean (SD) | 17 (27) | 17 (26) |
| - JSN score, mean (SD) | 19 (26) | 18 (25) |
*Based on prednisone equivalent.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease‑modifying antirheumatic drug; CCP=cyclic citrullinated peptide; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; EOW=every other week; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C‑reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; NSAID=nonsteroidal anti-inflammatory drug; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once per day; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
US-RNQ-230377
SELECT-BEYOND
Adults with moderately to severely active RA who had an inadequate response or intolerance to bDMARDs1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aStarting at Week 24, initiation of or change in corticosteroids, NSAIDs, acetaminophen, and csDMARDs was permitted. Patients not achieving response criteria ≥20% improvement in SJC and TJC at 2 consecutive visits were removed from the study.4,5
bFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.8
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS6,7
At Week 12:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS7
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS6
| MEAN (SD) OR N (%) | PBO + csDMARDs n=169 | RINVOQ 15 mg + csDMARDs n=164 |
|---|---|---|
| Female, n (%) | 143 (85) | 137 (84) |
| Age (years), mean (SD) | 57.6 (11.4) | 56.3 (11.3) |
| Duration since RA diagnosis (years), mean (SD) | 14.5 (9.2) | 12.4 (9.4) |
| RF+ and/or ACPA+, n (%) | 128 (76) | 131 (80) |
| csDMARD use at baseline* | ||
| - MTX alone,† n (%) | 122 (73) | 118 (73) |
| - MTX plus other csDMARD,‡ n (%) | 17 (10) | 19 (12) |
| - MTX dose§ (mg), mean (SD) | 16.6 (4.7) | 17.3 (4.6) |
| - csDMARD other than MTX, n (%) | 29 (17) | 24 (15) |
| - Missing, n | 1 | 3 |
| Prior bDMARD exposure | ||
| - 1, n (%) | 83 (49) | 86 (52) |
| - 2, n (%) | 46 (27) | 40 (24) |
| - ≥3, n (%) | 40 (24) | 38 (23) |
| Inadequate response or intolerance to ≥1 anti-TNF drug | 152 (90) | 146 (89) |
| - Lack of efficacy with ≥1 bDMARD | 159 (94) | 146 (89) |
| - Lack of efficacy with ≥1 anti-IL-6 | 30 (18) | 27 (16) |
| Oral glucocorticoid use, n (%) | 74 (44) | 83 (51) |
| - Oral glucocorticoid dosell (mg), mean (SD) | 6.3 (2.4) | 5.7 (2.4) |
| TJC68, mean (SD) | 28.5 (15.3) | 27.8 (16.3) |
| SJC66, mean (SD) | 16.3 (9.6) | 17.0 (10.8) |
| PtGA (0-100 mm VAS), mean (SD) | 66.3 (22.7) | 67.2 (19.6) |
| PhGA (0-100 mm VAS), mean (SD) | 66.9 (16.9) | 68.7 (16.6) |
| Pain (0-100 mm VAS), mean (SD) | 68.9 (21.0) | 68.2 (19.8) |
| hsCRP (mg/L), mean (SD) | 16.3 (21.1) | 16.2 (18.6) |
| DAS28-CRP, mean (SD) | 5.8 (1.0) | 5.9 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.6) | 1.7 (0.6) |
| CDAI, mean (SD) | 41.0 (13.3) | 41.7 (13.3) |
| SDAI, mean (SD) | 42.6 (13.9) | 43.3 (13.8) |
*Oral or parenteral methotrexate (7.5-25 mg per week).
†Data available for 168 patients receiving placebo and 161 patients receiving RINVOQ 15 mg.
‡All combinations allowed except MTX and leflunomide.
§Mean MTX dose calculated only for patients receiving MTX.
‖Based on prednisone equivalent.
ACPA=anti‑citrullinated protein antibodies; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease‑modifying antirheumatic drug; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease‑modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high‑sensitivity C‑reactive protein; IL-6=interleukin 6; IR=intolerance or inadequate response; LDA=low disease activity; MTX=methotrexate; NSAID=nonsteroidal anti‑inflammatory drug; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36‑item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-EARLY
Adults with moderately to severely active RA who were MTX‑naïve1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aInitially 947 patients were randomized in the study, but 2 patients were never dosed.
bX-ray images of hands and feet obtained at these time points.2
cStarting at Week 12, patients with ≤20% improvement in TJC and SJC compared to baseline at 2 consecutive visits continued blinded therapy and optimized background RA medications (corticosteroids, NSAIDs, and/or low‑potency analgesics).3,4
dAt Week 26, patients with CDAI ≤2.8 continued their original study drug; background medications (NSAIDs, corticosteroids, and/or low‑potency analgesics, and csDMARDs) were optimized in patients with CDAI >2.8 but ≥20% improvement in TJC and SJC; among patients with CDAI >2.8 and <20% improvement in TJC and SJC, RINVOQ 15 mg or upadacitinib 30 mg were added by re‑randomization according to 1:1 ratio for those initially randomized to MTX, and MTX was added for those initially randomized to RINVOQ 15 mg or upadacitinib 30 mg.2,5
eStarting at Week 48, patients who did not achieve ≥20% improvement in both TJC and SJC at 2 consecutive visits were removed from the study. Initiation of or change in background RA medications (NSAIDs, corticosteroids, low-potency analgesics, and csDMARDs; not all patients received background MTX) is allowed at anytime during Period 2.6
fFollowing a protocol amendment, all patients in the long-term extension who were previously receiving UPA 30 mg received RINVOQ 15 mg.11
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS3,8
At Week 12:
At Week 24:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS2,3,9,10
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | MTX n=314 | RINVOQ 15 mg QD n=317 |
|---|---|---|
| Female, n (%) | 240 (76) | 241 (76) |
| Age (years), mean (SD) | 53.3 (12.9) | 51.9 (12.6) |
| Duration since RA diagnosis (years), mean (SD) | 2.6 (5.1) | 2.9 (5.4) |
| RF+ and/or ACPA+, n (%) | 255 (81) | 279 (88) |
| MTX exposure, n (%) | 19 (6) | 30 (9.5) |
| csDMARD exposure, n (%) | 79 (25) | 80 (25) |
| Oral glucocorticoid use, n (%) | 163 (52) | 147 (46) |
| - Dose (mg),* mean (SD) | 6.4 (2.4) | 6.4 (3.1) |
| TJC68, mean (SD) | 26.4 (16.2) | 25.4 (14.4) |
| SJC66, mean (SD) | 16.9 (10.6) | 16.9 (10.4) |
| PtGA (0-100 mm VAS), mean (SD) | 65.8 (21.5) | 66.6 (22.0) |
| PhGA (0-100 mm VAS), mean (SD) | 68.7 (16.5) | 67.1 (17.0) |
| Pain (0-100 mm VAS), mean (SD) | 65.7 (21.5) | 68.4 (20.6) |
| hsCRP (mg/L), mean (SD) | 21.2 (22.1) | 23.0 (27.4) |
| DAS28-CRP, mean (SD) | 5.9 (1.0) | 5.9 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.7) | 1.6 (0.7) |
| CDAI, mean (SD) | 40.5 (13.3) | 40.4 (13.3) |
| SDAI, mean (SD) | 42.6 (14.0) | 42.7 (13.9) |
| mTSS, mean (SD) | 13.3 (30.6) | 18.1 (38.2) |
| - Erosion Score, mean (SD) | 6.1 (15.5) | 8.6 (19.3) |
| - Joint Space Narrowing Score, mean (SD) | 7.2 (16.2) | 9.6 (20.1) |
*Prednisone equivalent dose in patients receiving oral glucocorticoids at baseline.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C-reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; HAQ-DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C-reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; NSAID=nonsteroidal anti-inflammatory drug; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF-36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-MONOTHERAPY
Adults with moderately to severely active RA who had an inadequate response to MTX1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aPatients on cMTX were randomized to receive either RINVOQ 15 mg or upadacitinib 30 mg at Week 14.3
bStarting at Week 26, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDs, acetaminophen, or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.5
cFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.4
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS3,6
At Week 14:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS3,6
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | cMTX n=216 | RINVOQ 15 mg QD n=217 |
|---|---|---|
| Female, n (%) | 179 (83) | 174 (80) |
| Age (years), mean (SD) | 55.3 (11.1) | 54.5 (12.2) |
| Duration since RA diagnosis (years), mean (SD) | 5.8 (6.6) | 7.5 (8.9) |
| RF+ and/or ACPA+, n (%) | 169 (78) | 172 (79) |
| Prior MTX dose* (mg/week), mean (SD) | 16.7 (4.4) | 16.8 (4.2) |
| Prior MTX duration (years), mean (SD) | 3.3 (3.9) | 3.8 (4.8) |
| Oral glucocorticoid use, n (%) | 115 (53) | 114 (53) |
| - Oral glucocorticoid dose† (mg), mean (SD) | 6.2 (2.6) | 6.1 (2.5) |
| TJC68, mean (SD) | 25.2 (16.0) | 24.5 (15.1) |
| SJC66, mean (SD) | 16.9 (11.5) | 16.4 (10.9) |
| PtGA (0-100 mm VAS), mean (SD) | 59.6 (21.8) | 62.2 (22.3) |
| PhGA (0-100 mm VAS), mean (SD) | 62.1 (17.5) | 65.7 (18.5) |
| Pain (0-100 mm VAS), mean (SD) | 62.5 (21.3) | 62.3 (22.5) |
| hsCRP (mg/L), mean (SD) | 14.5 (17.3) | 14.0 (16.5) |
| DAS28-CRP, mean (SD) | 5.6 (1.0) | 5.6 (0.9) |
| HAQ-DI, mean (SD) | 1.5 (0.7) | 1.5 (0.7) |
| CDAI, mean (SD) | 37.8 (14.4) | 38.0 (13.1) |
| SDAI, mean (SD) | 39.2 (14.6) | 39.4 (13.4) |
*Prior to receiving study drug. In the control arm, patients continued prior MTX dose as blinded study drug.3
†Prednisone equivalent.
ACPA=anti‑citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=Clinical Disease Activity Index; cMTX=continuous methotrexate; csDMARD=conventional synthetic disease‑modifying antirheumatic drug; CR=clinical remission; CRP=C‑reactive protein; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C‑reactive protein; IR=intolerance or inadequate response; LDA=low disease activity; MTX=methotrexate; NSAID=nonsteroidal anti‑inflammatory drug; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-NEXT
Adults with moderately to severely active RA who had an inadequate response to csDMARD(s)1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aStarting at Week 24, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDs, acetaminophen, or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.4
bFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.7
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS5,6
At Week 12:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS6
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | PBO + csDMARDs n=221 | RINVOQ 15 mg QD + csDMARDs n=221 |
|---|---|---|
| Female, n (%) | 166 (75) | 182 (82) |
| Age (years), mean (SD) | 56.0 (12.2) | 55.3 (11.5) |
| Duration since RA diagnosis (years), mean (SD) | 7.2 (7.5) | 7.3 (7.9) |
| RF+ and/or ACPA+, n (%) | 181 (82) | 184 (83) |
| csDMARD use at baseline | ||
| - MTX alone, n (%) | 141 (64) | 122 (55) |
| - MTX plus other csDMARD, n (%) | 49 (22) | 47 (21) |
| - csDMARD other than MTX, n (%) | 30 (14) | 51 (23) |
| - Missing, n (%) | 1 (<1) | 1 (<1) |
| Prior bDMARD exposure, n (%) | 29 (13) | 27 (12) |
| Oral glucocorticoid use, n (%) | 106 (48) | 96 (43) |
| - Oral glucocorticoid dose* (mg), mean (SD) | 6.3 (2.6) | 6.0 (2.4) |
| TJC68, mean (SD) | 24.7 (15.0) | 25.2 (13.8) |
| SJC66, mean (SD) | 15.4 (9.2) | 16.0 (10.0) |
| PtGA (0-100 mm VAS), mean (SD) | 60.3 (20.5) | 63.1 (21.9) |
| PhGA (0-100 mm VAS), mean (SD) | 64.4 (17.7) | 64.3 (16.2) |
| Pain (0-100 mm VAS), mean (SD) | 61.5 (20.8) | 64.1 (19.5) |
| hsCRP (mg/L), mean (SD) | 12.6 (14.0) | 16.6 (19.2) |
| DAS28-CRP, mean (SD) | 5.6 (0.8) | 5.7 (1.0) |
| HAQ-DI, mean (SD) | 1.4 (0.6) | 1.5 (0.6) |
| CDAI, mean (SD) | 37.8 (11.8) | 38.3 (11.9) |
| SDAI, mean (SD) | 39.0 (11.9) | 39.9 (12.5) |
*Based on prednisone equivalent.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease-modifying antirheumatic drug; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C-reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; HAQ-DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C-reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; MTX=methotrexate; NSAIDs=nonsteroidal anti-inflammatory drugs; PBO=placebo; PhGA=physician's global assessment of disease activity; PtGA=patient's global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-COMPARE
Adults with moderately to severely active RA who had an inadequate response to MTX1
RINVOQ is indicated for TNFi-IR patients1

aX-ray imaging was performed at these time points; Week 14 for nonresponder patients, who were rescued.2,3
bRescue criteria: At Weeks 14, 18, and 22, if <20% improvement in TJC and SJC vs baseline; at Week 26, all remaining PBO patients were switched to RINVOQ, and patients receiving RINVOQ or active comparator were switched to active comparator or RINVOQ, respectively, if CDAI >10.2
cStarting at Week 26, initiation or change in background RA medication(s), including corticosteroids, NSAIDs, or acetaminophen was permitted.4
dStarting at Week 48, patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.5
eAt Week 48, initiation or change in csDMARDs was allowed; however, not all patients received background MTX.5
fPatients continued treatment with UPA or active comparator in a blinded manner until the last patient completed the Week 48 visit and received open-label treatment thereafter.8
PRIMARY ENDPOINT1
SELECT RANKED SECONDARY ENDPOINTS2
At Week 12 vs placebo + MTX:
At Week 12 vs active comparator + MTX:
At Week 26 vs placebo + MTX:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS3,9,10
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
SELECT-COMPARE was not designed to evaluate the efficacy of active comparator + MTX vs placebo + MTX. No conclusions regarding this comparison can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | PBO + MTX n=651 | RINVOQ 15 mg QD + MTX n=651 |
|---|---|---|
| Female, n (%) | 512 (79) | 521 (80) |
| Age (years), mean (SD) | 54 (12) | 54 (12) |
| Duration since RA diagnosis (years), mean (SD) | 8 (8) | 8 (8) |
| RF+ and/or anti-CCP+, n (%) | 571 (88) | 566 (87) |
| MTX dose (mg/week), mean (SD) | 16.8 (3.8) | 17.0 (4.2) |
| Prior bDMARD use, n (%) | 63 (10) | 54 (8) |
| Oral glucocorticoid use, n (%) | 392 (60) | 388 (60) |
| - Dose (mg),* mean (SD) | 6.3 (2.4) | 6.2 (2.3) |
| TJC68, mean (SD) | 26 (14) | 26 (15) |
| SJC66, mean (SD) | 16 (9) | 17 (10) |
| PtGA (0-100 mm VAS), mean (SD) | 64 (21) | 64 (22) |
| PhGA (0-100 mm VAS), mean (SD) | 66 (18) | 66 (17) |
| Pain (0-100 mm VAS), mean (SD) | 65 (21) | 66 (21) |
| hsCRP (mg/L), mean (SD) | 18 (22) | 18 (22) |
| DAS28-CRP, mean (SD) | 5.8 (0.9) | 5.8 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.6) | 1.6 (0.6) |
| CDAI, mean (SD) | 40 (13) | 40 (13) |
| SDAI, mean (SD) | 41.8 (13.3) | 41.5 (13.6) |
| mTSS, mean (SD) | 36 (52) | 34 (50) |
| - JE score, mean (SD) | 17 (27) | 17 (26) |
| - JSN score, mean (SD) | 19 (26) | 18 (25) |
*Based on prednisone equivalent.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease‑modifying antirheumatic drug; CCP=cyclic citrullinated peptide; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; EOW=every other week; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C‑reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; NSAID=nonsteroidal anti-inflammatory drug; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once per day; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
US-RNQ-230377
SELECT-BEYOND
Adults with moderately to severely active RA who had an inadequate response or intolerance to bDMARDs1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aStarting at Week 24, initiation of or change in corticosteroids, NSAIDs, acetaminophen, and csDMARDs was permitted. Patients not achieving response criteria ≥20% improvement in SJC and TJC at 2 consecutive visits were removed from the study.4,5
bFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.8
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS6,7
At Week 12:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS7
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS6
| MEAN (SD) OR N (%) | PBO + csDMARDs n=169 | RINVOQ 15 mg + csDMARDs n=164 |
|---|---|---|
| Female, n (%) | 143 (85) | 137 (84) |
| Age (years), mean (SD) | 57.6 (11.4) | 56.3 (11.3) |
| Duration since RA diagnosis (years), mean (SD) | 14.5 (9.2) | 12.4 (9.4) |
| RF+ and/or ACPA+, n (%) | 128 (76) | 131 (80) |
| csDMARD use at baseline* | ||
| - MTX alone,† n (%) | 122 (73) | 118 (73) |
| - MTX plus other csDMARD,‡ n (%) | 17 (10) | 19 (12) |
| - MTX dose§ (mg), mean (SD) | 16.6 (4.7) | 17.3 (4.6) |
| - csDMARD other than MTX, n (%) | 29 (17) | 24 (15) |
| - Missing, n | 1 | 3 |
| Prior bDMARD exposure | ||
| - 1, n (%) | 83 (49) | 86 (52) |
| - 2, n (%) | 46 (27) | 40 (24) |
| - ≥3, n (%) | 40 (24) | 38 (23) |
| Inadequate response or intolerance to ≥1 anti-TNF drug | 152 (90) | 146 (89) |
| - Lack of efficacy with ≥1 bDMARD | 159 (94) | 146 (89) |
| - Lack of efficacy with ≥1 anti-IL-6 | 30 (18) | 27 (16) |
| Oral glucocorticoid use, n (%) | 74 (44) | 83 (51) |
| - Oral glucocorticoid dosell (mg), mean (SD) | 6.3 (2.4) | 5.7 (2.4) |
| TJC68, mean (SD) | 28.5 (15.3) | 27.8 (16.3) |
| SJC66, mean (SD) | 16.3 (9.6) | 17.0 (10.8) |
| PtGA (0-100 mm VAS), mean (SD) | 66.3 (22.7) | 67.2 (19.6) |
| PhGA (0-100 mm VAS), mean (SD) | 66.9 (16.9) | 68.7 (16.6) |
| Pain (0-100 mm VAS), mean (SD) | 68.9 (21.0) | 68.2 (19.8) |
| hsCRP (mg/L), mean (SD) | 16.3 (21.1) | 16.2 (18.6) |
| DAS28-CRP, mean (SD) | 5.8 (1.0) | 5.9 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.6) | 1.7 (0.6) |
| CDAI, mean (SD) | 41.0 (13.3) | 41.7 (13.3) |
| SDAI, mean (SD) | 42.6 (13.9) | 43.3 (13.8) |
*Oral or parenteral methotrexate (7.5-25 mg per week).
†Data available for 168 patients receiving placebo and 161 patients receiving RINVOQ 15 mg.
‡All combinations allowed except MTX and leflunomide.
§Mean MTX dose calculated only for patients receiving MTX.
‖Based on prednisone equivalent.
ACPA=anti‑citrullinated protein antibodies; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease‑modifying antirheumatic drug; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease‑modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high‑sensitivity C‑reactive protein; IL-6=interleukin 6; IR=intolerance or inadequate response; LDA=low disease activity; MTX=methotrexate; NSAID=nonsteroidal anti‑inflammatory drug; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36‑item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-EARLY
Adults with moderately to severely active RA who were MTX‑naïve1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aInitially 947 patients were randomized in the study, but 2 patients were never dosed.
bX-ray images of hands and feet obtained at these time points.2
cStarting at Week 12, patients with ≤20% improvement in TJC and SJC compared to baseline at 2 consecutive visits continued blinded therapy and optimized background RA medications (corticosteroids, NSAIDs, and/or low‑potency analgesics).3,4
dAt Week 26, patients with CDAI ≤2.8 continued their original study drug; background medications (NSAIDs, corticosteroids, and/or low‑potency analgesics, and csDMARDs) were optimized in patients with CDAI >2.8 but ≥20% improvement in TJC and SJC; among patients with CDAI >2.8 and <20% improvement in TJC and SJC, RINVOQ 15 mg or upadacitinib 30 mg were added by re‑randomization according to 1:1 ratio for those initially randomized to MTX, and MTX was added for those initially randomized to RINVOQ 15 mg or upadacitinib 30 mg.2,5
eStarting at Week 48, patients who did not achieve ≥20% improvement in both TJC and SJC at 2 consecutive visits were removed from the study. Initiation of or change in background RA medications (NSAIDs, corticosteroids, low-potency analgesics, and csDMARDs; not all patients received background MTX) is allowed at anytime during Period 2.6
fFollowing a protocol amendment, all patients in the long-term extension who were previously receiving UPA 30 mg received RINVOQ 15 mg.11
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS3,8
At Week 12:
At Week 24:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS2,3,9,10
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | MTX n=314 | RINVOQ 15 mg QD n=317 |
|---|---|---|
| Female, n (%) | 240 (76) | 241 (76) |
| Age (years), mean (SD) | 53.3 (12.9) | 51.9 (12.6) |
| Duration since RA diagnosis (years), mean (SD) | 2.6 (5.1) | 2.9 (5.4) |
| RF+ and/or ACPA+, n (%) | 255 (81) | 279 (88) |
| MTX exposure, n (%) | 19 (6) | 30 (9.5) |
| csDMARD exposure, n (%) | 79 (25) | 80 (25) |
| Oral glucocorticoid use, n (%) | 163 (52) | 147 (46) |
| - Dose (mg),* mean (SD) | 6.4 (2.4) | 6.4 (3.1) |
| TJC68, mean (SD) | 26.4 (16.2) | 25.4 (14.4) |
| SJC66, mean (SD) | 16.9 (10.6) | 16.9 (10.4) |
| PtGA (0-100 mm VAS), mean (SD) | 65.8 (21.5) | 66.6 (22.0) |
| PhGA (0-100 mm VAS), mean (SD) | 68.7 (16.5) | 67.1 (17.0) |
| Pain (0-100 mm VAS), mean (SD) | 65.7 (21.5) | 68.4 (20.6) |
| hsCRP (mg/L), mean (SD) | 21.2 (22.1) | 23.0 (27.4) |
| DAS28-CRP, mean (SD) | 5.9 (1.0) | 5.9 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.7) | 1.6 (0.7) |
| CDAI, mean (SD) | 40.5 (13.3) | 40.4 (13.3) |
| SDAI, mean (SD) | 42.6 (14.0) | 42.7 (13.9) |
| mTSS, mean (SD) | 13.3 (30.6) | 18.1 (38.2) |
| - Erosion Score, mean (SD) | 6.1 (15.5) | 8.6 (19.3) |
| - Joint Space Narrowing Score, mean (SD) | 7.2 (16.2) | 9.6 (20.1) |
*Prednisone equivalent dose in patients receiving oral glucocorticoids at baseline.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C-reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; HAQ-DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C-reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; NSAID=nonsteroidal anti-inflammatory drug; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF-36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-MONOTHERAPY
Adults with moderately to severely active RA who had an inadequate response to MTX1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aPatients on cMTX were randomized to receive either RINVOQ 15 mg or upadacitinib 30 mg at Week 14.3
bStarting at Week 26, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDs, acetaminophen, or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.5
cFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.4
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS3,6
At Week 14:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS3,6
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | cMTX n=216 | RINVOQ 15 mg QD n=217 |
|---|---|---|
| Female, n (%) | 179 (83) | 174 (80) |
| Age (years), mean (SD) | 55.3 (11.1) | 54.5 (12.2) |
| Duration since RA diagnosis (years), mean (SD) | 5.8 (6.6) | 7.5 (8.9) |
| RF+ and/or ACPA+, n (%) | 169 (78) | 172 (79) |
| Prior MTX dose* (mg/week), mean (SD) | 16.7 (4.4) | 16.8 (4.2) |
| Prior MTX duration (years), mean (SD) | 3.3 (3.9) | 3.8 (4.8) |
| Oral glucocorticoid use, n (%) | 115 (53) | 114 (53) |
| - Oral glucocorticoid dose† (mg), mean (SD) | 6.2 (2.6) | 6.1 (2.5) |
| TJC68, mean (SD) | 25.2 (16.0) | 24.5 (15.1) |
| SJC66, mean (SD) | 16.9 (11.5) | 16.4 (10.9) |
| PtGA (0-100 mm VAS), mean (SD) | 59.6 (21.8) | 62.2 (22.3) |
| PhGA (0-100 mm VAS), mean (SD) | 62.1 (17.5) | 65.7 (18.5) |
| Pain (0-100 mm VAS), mean (SD) | 62.5 (21.3) | 62.3 (22.5) |
| hsCRP (mg/L), mean (SD) | 14.5 (17.3) | 14.0 (16.5) |
| DAS28-CRP, mean (SD) | 5.6 (1.0) | 5.6 (0.9) |
| HAQ-DI, mean (SD) | 1.5 (0.7) | 1.5 (0.7) |
| CDAI, mean (SD) | 37.8 (14.4) | 38.0 (13.1) |
| SDAI, mean (SD) | 39.2 (14.6) | 39.4 (13.4) |
*Prior to receiving study drug. In the control arm, patients continued prior MTX dose as blinded study drug.3
†Prednisone equivalent.
ACPA=anti‑citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=Clinical Disease Activity Index; cMTX=continuous methotrexate; csDMARD=conventional synthetic disease‑modifying antirheumatic drug; CR=clinical remission; CRP=C‑reactive protein; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C‑reactive protein; IR=intolerance or inadequate response; LDA=low disease activity; MTX=methotrexate; NSAID=nonsteroidal anti‑inflammatory drug; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-NEXT
Adults with moderately to severely active RA who had an inadequate response to csDMARD(s)1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aStarting at Week 24, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDs, acetaminophen, or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.4
bFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.7
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS5,6
At Week 12:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS6
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | PBO + csDMARDs n=221 | RINVOQ 15 mg QD + csDMARDs n=221 |
|---|---|---|
| Female, n (%) | 166 (75) | 182 (82) |
| Age (years), mean (SD) | 56.0 (12.2) | 55.3 (11.5) |
| Duration since RA diagnosis (years), mean (SD) | 7.2 (7.5) | 7.3 (7.9) |
| RF+ and/or ACPA+, n (%) | 181 (82) | 184 (83) |
| csDMARD use at baseline | ||
| - MTX alone, n (%) | 141 (64) | 122 (55) |
| - MTX plus other csDMARD, n (%) | 49 (22) | 47 (21) |
| - csDMARD other than MTX, n (%) | 30 (14) | 51 (23) |
| - Missing, n (%) | 1 (<1) | 1 (<1) |
| Prior bDMARD exposure, n (%) | 29 (13) | 27 (12) |
| Oral glucocorticoid use, n (%) | 106 (48) | 96 (43) |
| - Oral glucocorticoid dose* (mg), mean (SD) | 6.3 (2.6) | 6.0 (2.4) |
| TJC68, mean (SD) | 24.7 (15.0) | 25.2 (13.8) |
| SJC66, mean (SD) | 15.4 (9.2) | 16.0 (10.0) |
| PtGA (0-100 mm VAS), mean (SD) | 60.3 (20.5) | 63.1 (21.9) |
| PhGA (0-100 mm VAS), mean (SD) | 64.4 (17.7) | 64.3 (16.2) |
| Pain (0-100 mm VAS), mean (SD) | 61.5 (20.8) | 64.1 (19.5) |
| hsCRP (mg/L), mean (SD) | 12.6 (14.0) | 16.6 (19.2) |
| DAS28-CRP, mean (SD) | 5.6 (0.8) | 5.7 (1.0) |
| HAQ-DI, mean (SD) | 1.4 (0.6) | 1.5 (0.6) |
| CDAI, mean (SD) | 37.8 (11.8) | 38.3 (11.9) |
| SDAI, mean (SD) | 39.0 (11.9) | 39.9 (12.5) |
*Based on prednisone equivalent.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease-modifying antirheumatic drug; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C-reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; HAQ-DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C-reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; MTX=methotrexate; NSAIDs=nonsteroidal anti-inflammatory drugs; PBO=placebo; PhGA=physician's global assessment of disease activity; PtGA=patient's global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-COMPARE
Adults with moderately to severely active RA who had an inadequate response to MTX1
RINVOQ is indicated for TNFi-IR patients1

aX-ray imaging was performed at these time points; Week 14 for nonresponder patients, who were rescued.2,3
bRescue criteria: At Weeks 14, 18, and 22, if <20% improvement in TJC and SJC vs baseline; at Week 26, all remaining PBO patients were switched to RINVOQ, and patients receiving RINVOQ or active comparator were switched to active comparator or RINVOQ, respectively, if CDAI >10.2
cStarting at Week 26, initiation or change in background RA medication(s), including corticosteroids, NSAIDs, or acetaminophen was permitted.4
dStarting at Week 48, patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.5
eAt Week 48, initiation or change in csDMARDs was allowed; however, not all patients received background MTX.5
fPatients continued treatment with UPA or active comparator in a blinded manner until the last patient completed the Week 48 visit and received open-label treatment thereafter.8
PRIMARY ENDPOINT1
SELECT RANKED SECONDARY ENDPOINTS2
At Week 12 vs placebo + MTX:
At Week 12 vs active comparator + MTX:
At Week 26 vs placebo + MTX:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS3,9,10
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
SELECT-COMPARE was not designed to evaluate the efficacy of active comparator + MTX vs placebo + MTX. No conclusions regarding this comparison can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | PBO + MTX n=651 | RINVOQ 15 mg QD + MTX n=651 |
|---|---|---|
| Female, n (%) | 512 (79) | 521 (80) |
| Age (years), mean (SD) | 54 (12) | 54 (12) |
| Duration since RA diagnosis (years), mean (SD) | 8 (8) | 8 (8) |
| RF+ and/or anti-CCP+, n (%) | 571 (88) | 566 (87) |
| MTX dose (mg/week), mean (SD) | 16.8 (3.8) | 17.0 (4.2) |
| Prior bDMARD use, n (%) | 63 (10) | 54 (8) |
| Oral glucocorticoid use, n (%) | 392 (60) | 388 (60) |
| - Dose (mg),* mean (SD) | 6.3 (2.4) | 6.2 (2.3) |
| TJC68, mean (SD) | 26 (14) | 26 (15) |
| SJC66, mean (SD) | 16 (9) | 17 (10) |
| PtGA (0-100 mm VAS), mean (SD) | 64 (21) | 64 (22) |
| PhGA (0-100 mm VAS), mean (SD) | 66 (18) | 66 (17) |
| Pain (0-100 mm VAS), mean (SD) | 65 (21) | 66 (21) |
| hsCRP (mg/L), mean (SD) | 18 (22) | 18 (22) |
| DAS28-CRP, mean (SD) | 5.8 (0.9) | 5.8 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.6) | 1.6 (0.6) |
| CDAI, mean (SD) | 40 (13) | 40 (13) |
| SDAI, mean (SD) | 41.8 (13.3) | 41.5 (13.6) |
| mTSS, mean (SD) | 36 (52) | 34 (50) |
| - JE score, mean (SD) | 17 (27) | 17 (26) |
| - JSN score, mean (SD) | 19 (26) | 18 (25) |
*Based on prednisone equivalent.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease‑modifying antirheumatic drug; CCP=cyclic citrullinated peptide; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; EOW=every other week; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C‑reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; NSAID=nonsteroidal anti-inflammatory drug; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once per day; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
US-RNQ-230377
SELECT-BEYOND
Adults with moderately to severely active RA who had an inadequate response or intolerance to bDMARDs1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aStarting at Week 24, initiation of or change in corticosteroids, NSAIDs, acetaminophen, and csDMARDs was permitted. Patients not achieving response criteria ≥20% improvement in SJC and TJC at 2 consecutive visits were removed from the study.4,5
bFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.8
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS6,7
At Week 12:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS7
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS6
| MEAN (SD) OR N (%) | PBO + csDMARDs n=169 | RINVOQ 15 mg + csDMARDs n=164 |
|---|---|---|
| Female, n (%) | 143 (85) | 137 (84) |
| Age (years), mean (SD) | 57.6 (11.4) | 56.3 (11.3) |
| Duration since RA diagnosis (years), mean (SD) | 14.5 (9.2) | 12.4 (9.4) |
| RF+ and/or ACPA+, n (%) | 128 (76) | 131 (80) |
| csDMARD use at baseline* | ||
| - MTX alone,† n (%) | 122 (73) | 118 (73) |
| - MTX plus other csDMARD,‡ n (%) | 17 (10) | 19 (12) |
| - MTX dose§ (mg), mean (SD) | 16.6 (4.7) | 17.3 (4.6) |
| - csDMARD other than MTX, n (%) | 29 (17) | 24 (15) |
| - Missing, n | 1 | 3 |
| Prior bDMARD exposure | ||
| - 1, n (%) | 83 (49) | 86 (52) |
| - 2, n (%) | 46 (27) | 40 (24) |
| - ≥3, n (%) | 40 (24) | 38 (23) |
| Inadequate response or intolerance to ≥1 anti-TNF drug | 152 (90) | 146 (89) |
| - Lack of efficacy with ≥1 bDMARD | 159 (94) | 146 (89) |
| - Lack of efficacy with ≥1 anti-IL-6 | 30 (18) | 27 (16) |
| Oral glucocorticoid use, n (%) | 74 (44) | 83 (51) |
| - Oral glucocorticoid dosell (mg), mean (SD) | 6.3 (2.4) | 5.7 (2.4) |
| TJC68, mean (SD) | 28.5 (15.3) | 27.8 (16.3) |
| SJC66, mean (SD) | 16.3 (9.6) | 17.0 (10.8) |
| PtGA (0-100 mm VAS), mean (SD) | 66.3 (22.7) | 67.2 (19.6) |
| PhGA (0-100 mm VAS), mean (SD) | 66.9 (16.9) | 68.7 (16.6) |
| Pain (0-100 mm VAS), mean (SD) | 68.9 (21.0) | 68.2 (19.8) |
| hsCRP (mg/L), mean (SD) | 16.3 (21.1) | 16.2 (18.6) |
| DAS28-CRP, mean (SD) | 5.8 (1.0) | 5.9 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.6) | 1.7 (0.6) |
| CDAI, mean (SD) | 41.0 (13.3) | 41.7 (13.3) |
| SDAI, mean (SD) | 42.6 (13.9) | 43.3 (13.8) |
*Oral or parenteral methotrexate (7.5-25 mg per week).
†Data available for 168 patients receiving placebo and 161 patients receiving RINVOQ 15 mg.
‡All combinations allowed except MTX and leflunomide.
§Mean MTX dose calculated only for patients receiving MTX.
‖Based on prednisone equivalent.
ACPA=anti‑citrullinated protein antibodies; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease‑modifying antirheumatic drug; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease‑modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high‑sensitivity C‑reactive protein; IL-6=interleukin 6; IR=intolerance or inadequate response; LDA=low disease activity; MTX=methotrexate; NSAID=nonsteroidal anti‑inflammatory drug; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36‑item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-EARLY
Adults with moderately to severely active RA who were MTX‑naïve1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aInitially 947 patients were randomized in the study, but 2 patients were never dosed.
bX-ray images of hands and feet obtained at these time points.2
cStarting at Week 12, patients with ≤20% improvement in TJC and SJC compared to baseline at 2 consecutive visits continued blinded therapy and optimized background RA medications (corticosteroids, NSAIDs, and/or low‑potency analgesics).3,4
dAt Week 26, patients with CDAI ≤2.8 continued their original study drug; background medications (NSAIDs, corticosteroids, and/or low‑potency analgesics, and csDMARDs) were optimized in patients with CDAI >2.8 but ≥20% improvement in TJC and SJC; among patients with CDAI >2.8 and <20% improvement in TJC and SJC, RINVOQ 15 mg or upadacitinib 30 mg were added by re‑randomization according to 1:1 ratio for those initially randomized to MTX, and MTX was added for those initially randomized to RINVOQ 15 mg or upadacitinib 30 mg.2,5
eStarting at Week 48, patients who did not achieve ≥20% improvement in both TJC and SJC at 2 consecutive visits were removed from the study. Initiation of or change in background RA medications (NSAIDs, corticosteroids, low-potency analgesics, and csDMARDs; not all patients received background MTX) is allowed at anytime during Period 2.6
fFollowing a protocol amendment, all patients in the long-term extension who were previously receiving UPA 30 mg received RINVOQ 15 mg.11
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS3,8
At Week 12:
At Week 24:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS2,3,9,10
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | MTX n=314 | RINVOQ 15 mg QD n=317 |
|---|---|---|
| Female, n (%) | 240 (76) | 241 (76) |
| Age (years), mean (SD) | 53.3 (12.9) | 51.9 (12.6) |
| Duration since RA diagnosis (years), mean (SD) | 2.6 (5.1) | 2.9 (5.4) |
| RF+ and/or ACPA+, n (%) | 255 (81) | 279 (88) |
| MTX exposure, n (%) | 19 (6) | 30 (9.5) |
| csDMARD exposure, n (%) | 79 (25) | 80 (25) |
| Oral glucocorticoid use, n (%) | 163 (52) | 147 (46) |
| - Dose (mg),* mean (SD) | 6.4 (2.4) | 6.4 (3.1) |
| TJC68, mean (SD) | 26.4 (16.2) | 25.4 (14.4) |
| SJC66, mean (SD) | 16.9 (10.6) | 16.9 (10.4) |
| PtGA (0-100 mm VAS), mean (SD) | 65.8 (21.5) | 66.6 (22.0) |
| PhGA (0-100 mm VAS), mean (SD) | 68.7 (16.5) | 67.1 (17.0) |
| Pain (0-100 mm VAS), mean (SD) | 65.7 (21.5) | 68.4 (20.6) |
| hsCRP (mg/L), mean (SD) | 21.2 (22.1) | 23.0 (27.4) |
| DAS28-CRP, mean (SD) | 5.9 (1.0) | 5.9 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.7) | 1.6 (0.7) |
| CDAI, mean (SD) | 40.5 (13.3) | 40.4 (13.3) |
| SDAI, mean (SD) | 42.6 (14.0) | 42.7 (13.9) |
| mTSS, mean (SD) | 13.3 (30.6) | 18.1 (38.2) |
| - Erosion Score, mean (SD) | 6.1 (15.5) | 8.6 (19.3) |
| - Joint Space Narrowing Score, mean (SD) | 7.2 (16.2) | 9.6 (20.1) |
*Prednisone equivalent dose in patients receiving oral glucocorticoids at baseline.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C-reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; HAQ-DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C-reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; NSAID=nonsteroidal anti-inflammatory drug; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF-36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-MONOTHERAPY
Adults with moderately to severely active RA who had an inadequate response to MTX1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aPatients on cMTX were randomized to receive either RINVOQ 15 mg or upadacitinib 30 mg at Week 14.3
bStarting at Week 26, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDs, acetaminophen, or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.5
cFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.4
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS3,6
At Week 14:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS3,6
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | cMTX n=216 | RINVOQ 15 mg QD n=217 |
|---|---|---|
| Female, n (%) | 179 (83) | 174 (80) |
| Age (years), mean (SD) | 55.3 (11.1) | 54.5 (12.2) |
| Duration since RA diagnosis (years), mean (SD) | 5.8 (6.6) | 7.5 (8.9) |
| RF+ and/or ACPA+, n (%) | 169 (78) | 172 (79) |
| Prior MTX dose* (mg/week), mean (SD) | 16.7 (4.4) | 16.8 (4.2) |
| Prior MTX duration (years), mean (SD) | 3.3 (3.9) | 3.8 (4.8) |
| Oral glucocorticoid use, n (%) | 115 (53) | 114 (53) |
| - Oral glucocorticoid dose† (mg), mean (SD) | 6.2 (2.6) | 6.1 (2.5) |
| TJC68, mean (SD) | 25.2 (16.0) | 24.5 (15.1) |
| SJC66, mean (SD) | 16.9 (11.5) | 16.4 (10.9) |
| PtGA (0-100 mm VAS), mean (SD) | 59.6 (21.8) | 62.2 (22.3) |
| PhGA (0-100 mm VAS), mean (SD) | 62.1 (17.5) | 65.7 (18.5) |
| Pain (0-100 mm VAS), mean (SD) | 62.5 (21.3) | 62.3 (22.5) |
| hsCRP (mg/L), mean (SD) | 14.5 (17.3) | 14.0 (16.5) |
| DAS28-CRP, mean (SD) | 5.6 (1.0) | 5.6 (0.9) |
| HAQ-DI, mean (SD) | 1.5 (0.7) | 1.5 (0.7) |
| CDAI, mean (SD) | 37.8 (14.4) | 38.0 (13.1) |
| SDAI, mean (SD) | 39.2 (14.6) | 39.4 (13.4) |
*Prior to receiving study drug. In the control arm, patients continued prior MTX dose as blinded study drug.3
†Prednisone equivalent.
ACPA=anti‑citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=Clinical Disease Activity Index; cMTX=continuous methotrexate; csDMARD=conventional synthetic disease‑modifying antirheumatic drug; CR=clinical remission; CRP=C‑reactive protein; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C‑reactive protein; IR=intolerance or inadequate response; LDA=low disease activity; MTX=methotrexate; NSAID=nonsteroidal anti‑inflammatory drug; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-NEXT
Adults with moderately to severely active RA who had an inadequate response to csDMARD(s)1
RINVOQ is indicated for TNFi-IR patients1

Upadacitinib 30 mg is not an approved dose.
aStarting at Week 24, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDs, acetaminophen, or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.4
bFollowing a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg.7
PRIMARY ENDPOINT1
RANKED SECONDARY ENDPOINTS5,6
At Week 12:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS6
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | PBO + csDMARDs n=221 | RINVOQ 15 mg QD + csDMARDs n=221 |
|---|---|---|
| Female, n (%) | 166 (75) | 182 (82) |
| Age (years), mean (SD) | 56.0 (12.2) | 55.3 (11.5) |
| Duration since RA diagnosis (years), mean (SD) | 7.2 (7.5) | 7.3 (7.9) |
| RF+ and/or ACPA+, n (%) | 181 (82) | 184 (83) |
| csDMARD use at baseline | ||
| - MTX alone, n (%) | 141 (64) | 122 (55) |
| - MTX plus other csDMARD, n (%) | 49 (22) | 47 (21) |
| - csDMARD other than MTX, n (%) | 30 (14) | 51 (23) |
| - Missing, n (%) | 1 (<1) | 1 (<1) |
| Prior bDMARD exposure, n (%) | 29 (13) | 27 (12) |
| Oral glucocorticoid use, n (%) | 106 (48) | 96 (43) |
| - Oral glucocorticoid dose* (mg), mean (SD) | 6.3 (2.6) | 6.0 (2.4) |
| TJC68, mean (SD) | 24.7 (15.0) | 25.2 (13.8) |
| SJC66, mean (SD) | 15.4 (9.2) | 16.0 (10.0) |
| PtGA (0-100 mm VAS), mean (SD) | 60.3 (20.5) | 63.1 (21.9) |
| PhGA (0-100 mm VAS), mean (SD) | 64.4 (17.7) | 64.3 (16.2) |
| Pain (0-100 mm VAS), mean (SD) | 61.5 (20.8) | 64.1 (19.5) |
| hsCRP (mg/L), mean (SD) | 12.6 (14.0) | 16.6 (19.2) |
| DAS28-CRP, mean (SD) | 5.6 (0.8) | 5.7 (1.0) |
| HAQ-DI, mean (SD) | 1.4 (0.6) | 1.5 (0.6) |
| CDAI, mean (SD) | 37.8 (11.8) | 38.3 (11.9) |
| SDAI, mean (SD) | 39.0 (11.9) | 39.9 (12.5) |
*Based on prednisone equivalent.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease-modifying antirheumatic drug; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C-reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; HAQ-DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C-reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; MTX=methotrexate; NSAIDs=nonsteroidal anti-inflammatory drugs; PBO=placebo; PhGA=physician's global assessment of disease activity; PtGA=patient's global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
SELECT-COMPARE
Adults with moderately to severely active RA who had an inadequate response to MTX1
RINVOQ is indicated for TNFi-IR patients1

aX-ray imaging was performed at these time points; Week 14 for nonresponder patients, who were rescued.2,3
bRescue criteria: At Weeks 14, 18, and 22, if <20% improvement in TJC and SJC vs baseline; at Week 26, all remaining PBO patients were switched to RINVOQ, and patients receiving RINVOQ or active comparator were switched to active comparator or RINVOQ, respectively, if CDAI >10.2
cStarting at Week 26, initiation or change in background RA medication(s), including corticosteroids, NSAIDs, or acetaminophen was permitted.4
dStarting at Week 48, patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study.5
eAt Week 48, initiation or change in csDMARDs was allowed; however, not all patients received background MTX.5
fPatients continued treatment with UPA or active comparator in a blinded manner until the last patient completed the Week 48 visit and received open-label treatment thereafter.8
PRIMARY ENDPOINT1
SELECT RANKED SECONDARY ENDPOINTS2
At Week 12 vs placebo + MTX:
At Week 12 vs active comparator + MTX:
At Week 26 vs placebo + MTX:
SELECT PRESPECIFIED NON-RANKED ENDPOINTS3,9,10
DATA LIMITATIONS
Prespecified non-ranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
SELECT-COMPARE was not designed to evaluate the efficacy of active comparator + MTX vs placebo + MTX. No conclusions regarding this comparison can be made.
BASELINE CHARACTERISTICS3
| MEAN (SD) OR N (%) | PBO + MTX n=651 | RINVOQ 15 mg QD + MTX n=651 |
|---|---|---|
| Female, n (%) | 512 (79) | 521 (80) |
| Age (years), mean (SD) | 54 (12) | 54 (12) |
| Duration since RA diagnosis (years), mean (SD) | 8 (8) | 8 (8) |
| RF+ and/or anti-CCP+, n (%) | 571 (88) | 566 (87) |
| MTX dose (mg/week), mean (SD) | 16.8 (3.8) | 17.0 (4.2) |
| Prior bDMARD use, n (%) | 63 (10) | 54 (8) |
| Oral glucocorticoid use, n (%) | 392 (60) | 388 (60) |
| - Dose (mg),* mean (SD) | 6.3 (2.4) | 6.2 (2.3) |
| TJC68, mean (SD) | 26 (14) | 26 (15) |
| SJC66, mean (SD) | 16 (9) | 17 (10) |
| PtGA (0-100 mm VAS), mean (SD) | 64 (21) | 64 (22) |
| PhGA (0-100 mm VAS), mean (SD) | 66 (18) | 66 (17) |
| Pain (0-100 mm VAS), mean (SD) | 65 (21) | 66 (21) |
| hsCRP (mg/L), mean (SD) | 18 (22) | 18 (22) |
| DAS28-CRP, mean (SD) | 5.8 (0.9) | 5.8 (1.0) |
| HAQ-DI, mean (SD) | 1.6 (0.6) | 1.6 (0.6) |
| CDAI, mean (SD) | 40 (13) | 40 (13) |
| SDAI, mean (SD) | 41.8 (13.3) | 41.5 (13.6) |
| mTSS, mean (SD) | 36 (52) | 34 (50) |
| - JE score, mean (SD) | 17 (27) | 17 (26) |
| - JSN score, mean (SD) | 19 (26) | 18 (25) |
*Based on prednisone equivalent.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biologic disease‑modifying antirheumatic drug; CCP=cyclic citrullinated peptide; CDAI=Clinical Disease Activity Index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint disease activity score using C-reactive protein; DAS28-ESR=28-joint disease activity score using erythrocyte sedimentation rate; EOW=every other week; ESR=erythrocyte sedimentation rate; HAQ‑DI=Health Assessment Questionnaire Disability Index; hsCRP=high-sensitivity C‑reactive protein; IR=intolerance or inadequate response; JE=joint erosion; JSN=joint space narrowing; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; NSAID=nonsteroidal anti-inflammatory drug; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once per day; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=Simplified Disease Activity Index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; UPA=upadacitinib; VAS=visual analog scale.
REFERENCES:
US-RNQ-230377
ACR response criteria incorporate physician and patient assessments, as well as acute inflammatory, pain, and physical function measures.
PARAMETER |
SCORE |
|---|---|
| Number of tender joints | 0-68 scale |
| Number of swollen joints | 0-66 scale |
| Physician's global assessment of disease activity | 0-100 mm visual analog scale; 0=no disease activity 100=extreme disease activity |
| Patient's global assessment of disease activity | |
| Patient's assessment of pain | 0-100 mm visual analog scale; 0=no pain, 100=severe pain |
| HAQ-DI | 0-3; 0=no difficulty, 3=unable to perform activity Measures the patient's ability to perform the following: dress/groom, arise, eat, walk, reach, grip, maintain hygiene, and maintain daily activity |
| CRP (mg/dL) | Normal is <0.8 mg/dL |
ACR=American College of Rheumatology; CRP=C-reactive protein; HAQ‑Dl=Health Assessment Questionnaire Disability Index.
REFERENCES:
US-RNQ-230377
RINVOQ vs
HUMIRA® (adalimumab)
SELECT-SWITCH Study in RA
Explore head-to-head data for RINVOQ vs HUMIRA in moderate to severe rheumatoid arthritis patients who have had an inadequate response or intolerance to 1 TNFi.
aTEAE: treatment-emergent adverse event, defined as an adverse event with onset on or after first dose of study drug and up to 30 days after last dose of RINVOQ or placebo.1
bRates shown are n/100 PY=number of subjects with at least 1 event per 100 PY.1
cReported as abnormal lymphocyte morphology and not confirmed to be lymphoma.1
dMACE defined as cardiovascular death, myocardial infarction, and stroke.1
eVTE: venous thromboembolism includes deep vein thrombosis (DVT) and pulmonary embolism (PE).1
AESI: adverse event of special interest, defined as an adverse event with onset on or after first dose of study drug and up to 30 days after last dose of RINVOQ.
AD=atopic dermatitis; AS=ankylosing spondylitis; CD=Crohn's disease; E/100 PY=events per 100 patient‑years; pJIA=polyarticular juvenile idiopathic arthritis; MACE=major adverse cardiovascular event; NMSC=nonmelanoma skin cancer; nr-axSpA=non-radiographic axial spondyloarthritis; PsA=psoriatic arthritis; PY=patient-year; RA=rheumatoid arthritis; TB=tuberculosis; UC=ulcerative colitis; VTE=venous thromboembolism.
REFERENCE:
US-RNQ-230377
By selecting "Yes" below, you certify that you are a Healthcare Professional and that you wish to proceed to the Healthcare Professionals Only section on the AbbVie Medical Information site. Products or treatments described on this site are available in the US but may not be available in all other countries. I am a licensed Healthcare Professional and wish to proceed to the Healthcare Professionals Only AbbVie Medical Information Site.
Conversely, the presence of this link does not imply the linked site's endorsement of rinvoqhcp.com or AbbVie.
Do you wish to leave this site?
US-RNQ-230377
You are leaving an AbbVie website and connecting to a site that is not under the control of AbbVie. AbbVie is not responsible for the contents of any such site or any further links from such site. AbbVie is providing these links to you only as a convenience and the inclusion of any link does not imply the endorsement of the linked site by AbbVie. You should also be aware that the linked site may be governed by its own set of terms and conditions and privacy policy for which AbbVie has no responsibility.
Conversely, the presence of this link does not imply the linked site's endorsement of rinvoqhcp.com or AbbVie.
INDICATIONS & IMPORTANT SAFETY INFORMATION: WARNING: Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis
INDICATIONS & IMPORTANT SAFETY INFORMATION: WARNING: Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis
INDICATIONS & IMPORTANT SAFETY INFORMATION: WARNING: Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis
INDICATIONS & IMPORTANT SAFETY INFORMATION: WARNING: Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis
RINVOQ is indicated for the treatment of:
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biologic disease-modifying antirheumatic drugs (bDMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine.
Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other immunosuppressants.
Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biological therapies for UC or CD, or with potent immunosuppressants such as azathioprine and cyclosporine.
RINVOQ/RINVOQ LQ is indicated for the treatment of:
Limitations of Use: RINVOQ/RINVOQ LQ is not recommended for use in combination with other JAK inhibitors, bDMARDs, or with potent immunosuppressants such as azathioprine and cyclosporine.
Patients treated with RINVOQ* are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids. If a serious infection develops, interrupt RINVOQ until the infection is controlled.
Reported infections include:
Carefully consider the risks and benefits of treatment with RINVOQ prior to initiating therapy in patients with chronic or recurrent infection. Monitor patients closely for the development of signs and symptoms of infection during and after treatment with RINVOQ, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy.
In a large, randomized, postmarketing safety study comparing another Janus kinase (JAK) inhibitor with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients ≥50 years old with at least one cardiovascular (CV) risk factor, a higher rate of all-cause mortality, including sudden CV death, was observed with the JAK inhibitor. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ.
Lymphoma and other malignancies have been observed in patients treated with RINVOQ.
In a large, randomized, postmarketing safety study comparing another JAK inhibitor with TNF blockers in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]), lymphomas, and lung cancer (in current or past smokers) was observed with the JAK inhibitor. Patients who are current or past smokers are at additional increased risk.
With RINVOQ, consider the benefits and risks for the individual patient prior to initiating or continuing therapy, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers. NMSCs have been reported in patients treated with RINVOQ. Periodic skin examination is recommended for patients who are at increased risk for skin cancer. Advise patients to limit sunlight exposure by wearing protective clothing and using sunscreen.
In a large, randomized, postmarketing study comparing another JAK inhibitor with TNF blockers in RA patients ≥50 years old with at least one CV risk factor, a higher rate of MACE (defined as cardiovascular death, myocardial infarction, and stroke) was observed with the JAK inhibitor. Patients who are current or past smokers are at additional increased risk. Discontinue RINVOQ in patients that have experienced a myocardial infarction or stroke.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ, particularly in patients who are current or past smokers and patients with other CV risk factors. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
Thromboses, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis, have occurred in patients treated for inflammatory conditions with JAK inhibitors, including RINVOQ. Many of these adverse events were serious and some resulted in death.
In a large, randomized, postmarketing study comparing another JAK inhibitor to TNF blockers in RA patients ≥50 years old with at least one CV risk factor, a higher rate of thrombosis was observed with the JAK inhibitor. Avoid RINVOQ in patients at risk. Patients with symptoms of thrombosis should discontinue RINVOQ and be promptly evaluated.
RINVOQ is contraindicated in patients with known hypersensitivity to upadacitinib or any of its excipients. Serious hypersensitivity reactions, such as anaphylaxis and angioedema, were reported in patients receiving RINVOQ in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue RINVOQ and institute appropriate therapy.
Gastrointestinal (GI) perforations have been reported in clinical trials with RINVOQ. Monitor RINVOQ-treated patients who may be at risk for GI perforation (e.g., patients with a history of diverticulitis and patients taking NSAIDs or corticosteroids). Promptly evaluate patients presenting with new onset abdominal pain for early identification of GI perforation.
Neutropenia
Treatment with RINVOQ was associated with an increased incidence of neutropenia (absolute neutrophil count [ANC] <1000 cells/mm3). Treatment with RINVOQ is not recommended in patients with an ANC <1000 cells/mm3. Evaluate neutrophil counts at baseline and thereafter according to routine patient management.
Lymphopenia
Absolute lymphocyte counts (ALC) <500 cells/mm3 were reported in RINVOQ-treated patients. Treatment with RINVOQ is not recommended in patients with an ALC <500 cells/mm3. Evaluate at baseline and thereafter according to routine patient management.
Anemia
Decreases in hemoglobin levels to <8 g/dL were reported in RINVOQ-treated patients. Treatment should not be initiated or should be interrupted in patients with hemoglobin levels <8 g/dL. Evaluate at baseline and thereafter according to routine patient management.
Lipids
Treatment with RINVOQ was associated with increases in lipid parameters, including total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol. Manage patients according to clinical guidelines for the management of hyperlipidemia. Evaluate patients 12 weeks after initiation of treatment and thereafter according to the clinical guidelines for hyperlipidemia.
Liver enzyme elevations
Treatment with RINVOQ was associated with increased incidence of liver enzyme elevation compared to placebo. Evaluate at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. If increases in aspartate aminotransferase (AST) or alanine aminotransferase (ALT) are observed during routine patient management and drug-induced liver injury is suspected, RINVOQ should be interrupted until this diagnosis is excluded.
Based on findings in animal studies, RINVOQ may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with RINVOQ and for 4 weeks after the final dose. Verify pregnancy status of females of reproductive potential prior to starting treatment with RINVOQ.
Avoid use of live vaccines during, or immediately prior to, RINVOQ therapy. Prior to initiating RINVOQ, patients should be brought up to date on all immunizations, including prophylactic varicella zoster or herpes zoster vaccinations, in agreement with current immunization guidelines.
Reports of medication residue in stool or ostomy output have occurred in patients taking RINVOQ. Most reports described anatomic or functional GI conditions with shortened GI transit times. Instruct patients to contact their healthcare provider if medication residue is observed repeatedly. Monitor patients clinically and consider alternative treatment if there is an inadequate therapeutic response.
There are no data on the presence of RINVOQ in human milk, the effects on the breastfed infant, or the effects on milk production. Available data in animals have shown the excretion of RINVOQ in milk. Advise patients that breastfeeding is not recommended during treatment with RINVOQ and for 6 days after the last dose.
RINVOQ is not recommended for use in patients with severe hepatic impairment.
The most common adverse reactions in RINVOQ clinical trials were upper respiratory tract infections, herpes zoster, herpes simplex, bronchitis, nausea, cough, pyrexia, acne, headache, peripheral edema, increased blood creatine phosphokinase, hypersensitivity, folliculitis, abdominal pain, increased weight, influenza, fatigue, neutropenia, myalgia, influenza-like illness, elevated liver enzymes, rash, and anemia.
Inform patients that retinal detachment has been reported in clinical trials with RINVOQ. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision while receiving RINVOQ.
Dosage Forms and Strengths: RINVOQ is available in 15 mg, 30 mg, and 45 mg extended-release tablets. RINVOQ LQ is available in a 1 mg/mL oral solution.
*Unless otherwise stated, “RINVOQ” in the IMPORTANT SAFETY INFORMATION refers to RINVOQ and RINVOQ LQ.
US-RNQ-250017
Please see full Prescribing Information.



