DURABLE REMISSION
WITH RAPID STEROID REDUCTION
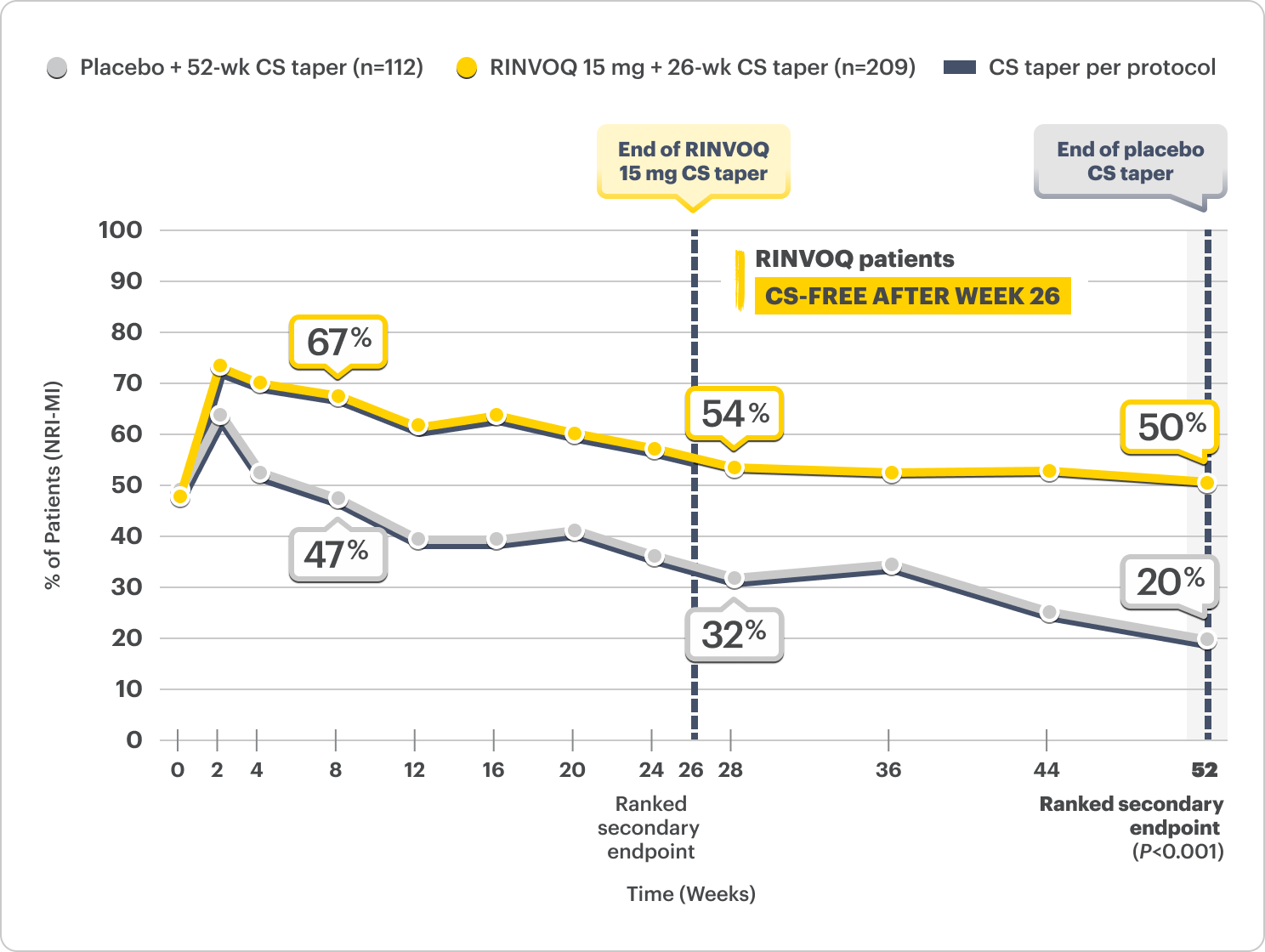
GCA patients on a 26-wk CS taper achieved sustained remission from Week 12 through 52 (primary endpoint).1,2
CS=corticosteroid; GCA=giant cell arteritis.
INDICATION
RINVOQ is indicated for the treatment of adults with giant cell arteritis (GCA).
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biologic disease-modifying antirheumatic drugs (bDMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine.
NRI Data From SELECT-GCA1,2
RINVOQ 15 mg + 26-wk CS taper (n=209), Placebo + 52-wk CS taper (n=112)
Sustained Remission
46% vs 29% placebo + 52-wk CS taper from Week 12 through 52*
PRIMARY ENDPOINT
*P=0.002.
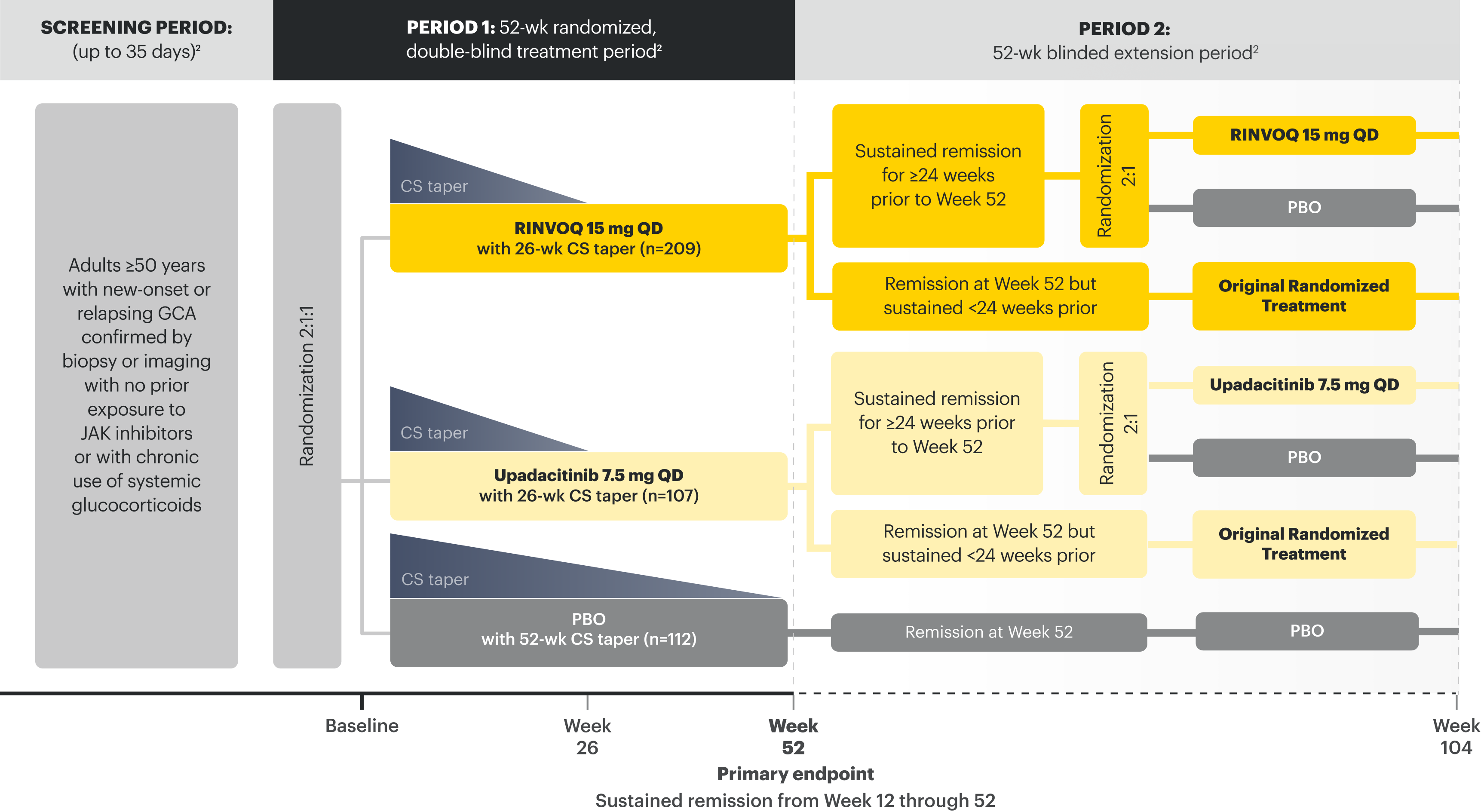
SELECT-GCA1-3:
52-wk, randomized, double-blind, placebo-controlled study of 428 adult patients age 50+ with GCA. Patients were randomized to receive RINVOQ 15 mg + 26-wk CS taper or placebo + 52-wk CS taper.
Sustained remission: Absence of GCA signs and symptoms from Week 12 through 52 and adherence to the protocol-defined CS-taper regimen.
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
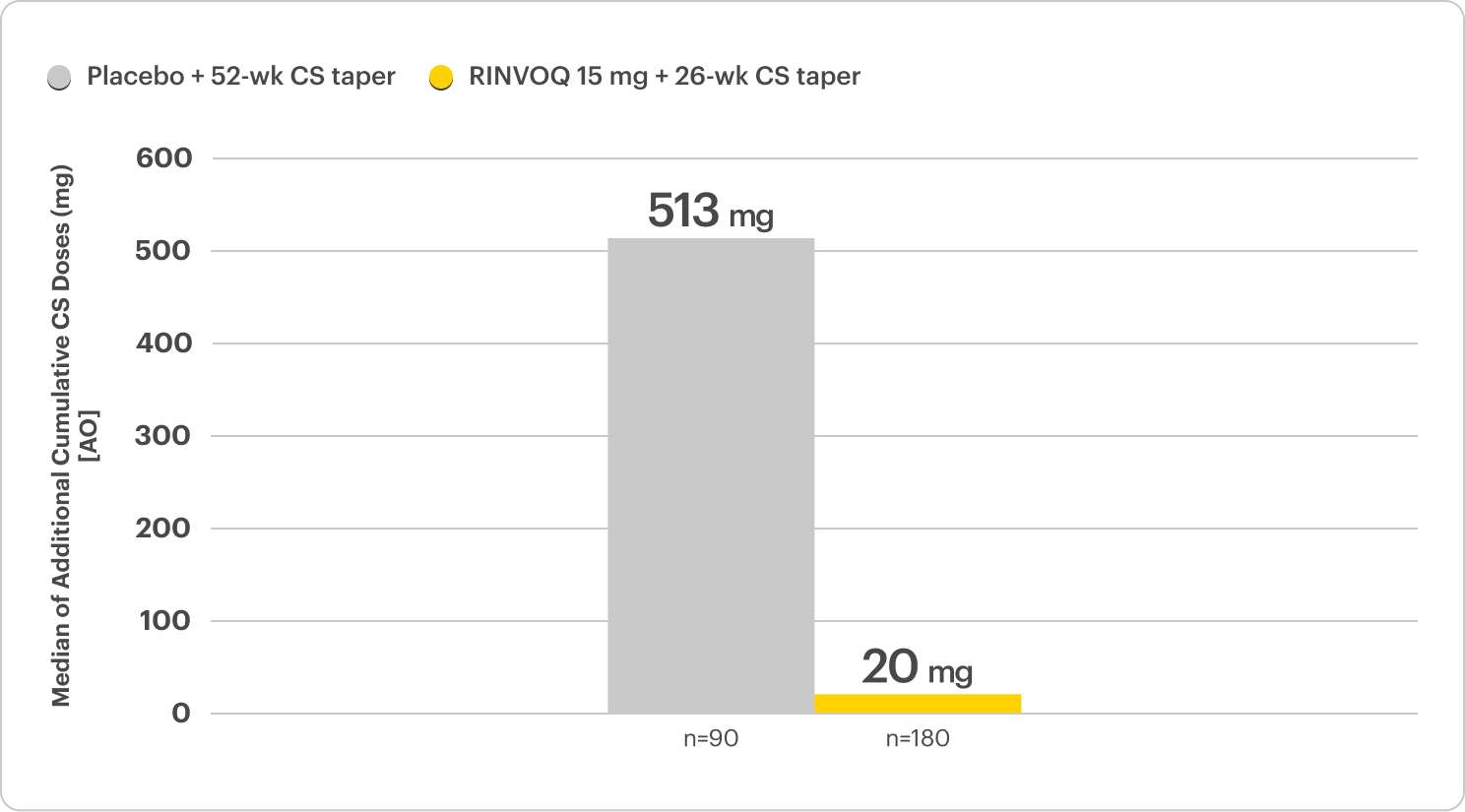
Rapid and Substantial Steroid Reduction
40% less cumulative steroid exposure at Week 52 with patients off steroids in half the time
on RINVOQ 15 mg + 26-wk CS taper vs placebo + 52-wk CS taper2,4
SELECT-GCA
ALL DATA ARE AS OBSERVED CASES
Median Cumulative CS Exposure

At Week 52, a substantially lower cumulative steroid dose was observed in the RINVOQ 15 mg + 26‑wk CS taper arm.
The cumulative CS exposure includes all CS use during the 52 weeks of the trial. This includes the CS dose per protocol and above protocol.
DATA LIMITATIONS: Data not labeled as ranked secondary endpoints were prespecified non-ranked endpoints not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
Steroid-Free Complete Remission at Week 52
Steroid-free remission achieved in a significantly higher proportion of patients at Week 522,4
SELECT-GCA
Complete Remission
COMPLETE REMISSION3:
Defined as achieving all of the following:
- Absence of GCA signs and symptoms
- Adherence to the protocol-defined CS-taper regimen
- Normalization of ESR (to ≤30 mm/hr), and
- Normalization of hsCRP (to <1 mg/dL)
DATA LIMITATIONS: Data not labeled as ranked secondary endpoints were prespecified non-ranked endpoints not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
Safety Considerations
Serious Infections: RINVOQ-treated patients are at increased risk of serious bacterial (including tuberculosis [TB]), fungal, viral, and opportunistic infections leading to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids.
Mortality: A higher rate of all-cause mortality, including sudden cardiovascular (CV) death, was observed with a Janus kinase inhibitor (JAKi) in a study comparing another JAKi with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients ≥50 years with ≥1 CV risk factor.
Malignancies: Malignancies have occurred in RINVOQ-treated patients. A higher rate of lymphomas and lung cancer (in current or past smokers) was observed with another JAKi when compared with TNF blockers in RA patients.
Major Adverse Cardiovascular Events: A higher rate of CV death, myocardial infarction, and stroke was observed with a JAKi in a study comparing another JAKi with TNF blockers in RA patients ≥50 years with ≥1 CV risk factor. History of smoking increases risk.
Thromboses: Deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated for inflammatory conditions with JAK inhibitors, including RINVOQ. A higher rate of thrombosis was observed with another JAKi when compared with TNF blockers in RA patients.
Hypersensitivity: RINVOQ is contraindicated in patients with hypersensitivity to RINVOQ or its excipients.
Other Serious Adverse Reactions: Hypersensitivity Reactions, Gastrointestinal Perforations, Laboratory Abnormalities, and Embryo-Fetal Toxicity.

Interested in the safety data for RINVOQ?
See RINVOQ’s safety data across clinical trials