WELL-STUDIED
SAFETY
PROFILE
Approved in 9 indications1
INDICATIONS
RINVOQ is indicated for the treatment of adults with:
- Moderately to severely active Crohn’s disease who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers.
- Moderately to severely active ulcerative colitis who have had an inadequate response or intolerance to one or more TNF blockers.
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biological therapies for Crohn’s disease or ulcerative colitis, or with potent immunosuppressants such as azathioprine and cyclosporine.
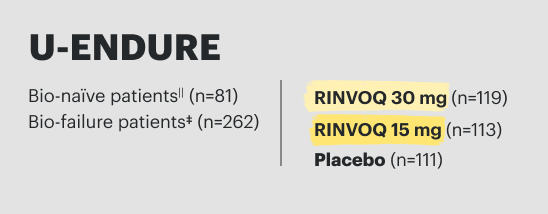
27 CLINICAL TRIALS
establishing a breadth of experience across indications1-5

12 years
of clinical experience* and approved
in 9 indications

>15,000
patients in global clinical trials across US-approved indications, including 2+ years in pJIA and pediatrics 12+ years in AD

Up to 6 years
of safety data in IBD

>45,000 patient-years
of exposure in clinical trials
*Clinical experience encompasses the time from first RINVOQ patient dosed in RA clinical trials to present.
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
Safety Profile
Safety Data Up to Week 52 at 15 mg and 30 mg doses
<<Swipe table to see more
| CROHN'S AT WEEK 526 | UC AT WEEK 527 | ||||||
|---|---|---|---|---|---|---|---|
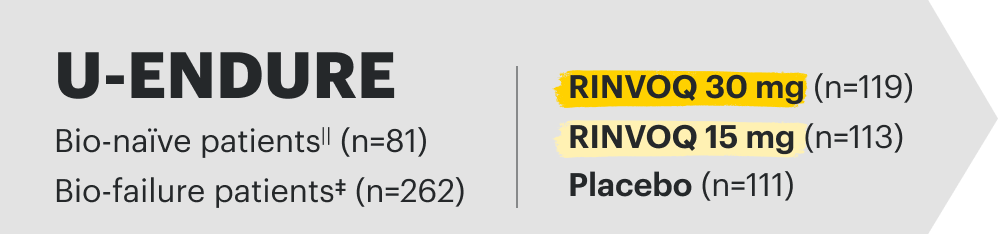
| U-ENDURE (CD) and U-ACHIEVE (UC): Maintenance data through Week 52 |
Placebo | RINVOQ 15 mg QD |
RINVOQ 30 mg QD |
Placebo | RINVOQ 15 mg QD |
RINVOQ 30 mg QD |
|
| ADVERSE EVENTS (AEs) | (N=223, PY=111.5) | (N=221, PY=153.7) | (N=229, PY=177.2) | (N=245, PY=135) | (N=250, PY=199.4) | (N=251, PY=218.5) | |
| TREATMENT-EMERGENT AEs | % | % | % | % | % | % | |
| Serious adverse event | 14.3 | 11.8 | 10.5 | 9.4 | 8.4 | 8.0 | |
| AE leading to discontinuation of study drug | 3.6 | 7.2 | 5.7 | 10.2 | 2.0 | 4.8 | |
| Death | 0 | 0 | 0 | 0 | 0 | 0 | |
| AEs OF SPECIAL INTEREST | % (N/100 PY) | % (N/100 PY) | % (N/100 PY) | % (N/100 PY) | % (N/100 PY) | % (N/100 PY) | |
| INFECTIONS | |||||||
| Serious infections | 4.5 (9.2) | 3.2 (4.6) | 4.8 (6.4) | 3.3 (6.0) | 3.6 (4.6) | 3.2 (3.7) | |
| Opportunistic infection (excluding TB and herpes zoster) |
0 | 0.5 (0.7) | 0.4 (0.6) | 0.8 (1.5) | 0.8 (1.0) | 0.8 (0.9) | |
| Active TB | 0 | 0 | 0 | 0 | 0 | 0 | |
| Herpes zoster | 2.2 (4.6) | 2.7 (4.0) | 5.7 (7.7) | 0 | 4.8 (6.2) | 5.6 (6.6) | |
| MALIGNANCY | |||||||
| Malignancy (excluding NMSC) | 0 | 0.5 (0.7) | 0.9 (1.1) | 0.4 (0.7) | 0.4 (0.5) | 0.8 (0.9) | |
| Lymphoma | 0 | 0 | 0 | 0 | 0 | 0 | |
| NMSC | 0 | 0 | 0 | 0 | 0 | 1.2 (1.4) | |
| CARDIOVASCULAR EVENTS | |||||||
| Adjudicated MACEa | 0 | 0 | 0 | 0.4 (0.7) | 0 | 0.4 (0.5) | |
| Adjudicated VTEb | 0 | 0 | 0 | 0 | 0.8 (1.0) | 0.8 (0.9) | |
| GASTROENTEROLOGICAL EVENTS | |||||||
| Adjudicated gastrointestinal perforations§ | 0.4 (0.9) | 0.5 (0.7) | 0.4 (0.6) | 0.4 (0.7) | 0 | 0 | |
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
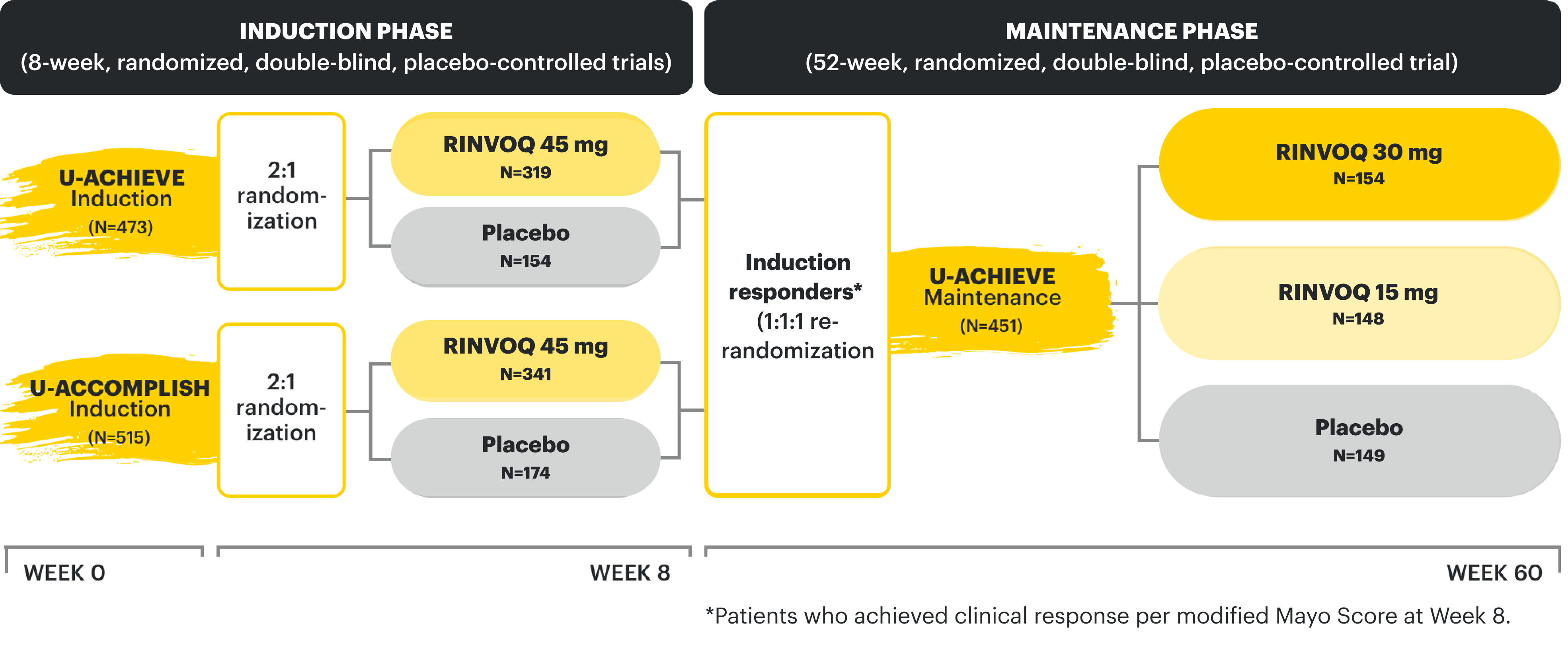
Week 52 CD Data: Patients responding to 12-week induction therapy with RINVOQ 45 mg randomized into the maintenance study. Data as of 1/2023.
Week 52 UC Data: Patients responding to 8-week induction therapy with RINVOQ 45 mg randomized into the maintenance study. Data as of 08/2023.
§In patients who lost response on placebo or RINVOQ 15 mg in U-ENDURE and were rescued with RINVOQ 30 mg (N=336): Gastrointestinal perforation was reported in 3 patients (1 per 100 patient-years) through long-term treatment.
Pooled safety data at 45 mg induction dose
<<Swipe table to see more
| CROHN'S AT WEEK 129,10 | UC AT WEEK 87,8 | ||||
|---|---|---|---|---|---|
| ADVERSE EVENTS OF SPECIAL INTEREST % (N/100 PY) unless otherwise noted |
Placebo (N=347) | RINVOQ 45 mg QD (N=674) | Placebo (N=378) | RINVOQ 45 mg QD (N=719) | |
| TREATMENT-EMERGENT AEs | % | % | % | % | |
| Serious adverse event | 8.4 | 8.0 | 5.8 | 3.1 | |
| AE leading to discontinuation of study drug | 5.5 | 4.9 | 7.1 | 2.4 | |
| Death | 0 | 0 | 0 | 0 | |
| AEs OF SPECIAL INTEREST | % (N/100 PY) | % (N/100 PY) | % (N/100 PY) | % (N/100 PY) | |
| INFECTIONS | |||||
| Serious infections | 1.7 (7.9) | 1.9 (8.7) | 1.3 (9.0) | 1.3 (8.2) | |
| Opportunistic infections (excluding TB and herpes zoster) |
0 | 0.3 (1.3) | 0.3 (1.8) | 0.4 (2.7) | |
| Active TB | 0 | 0 | 0 | 0 | |
| Herpes zoster | 0 | 2.2 (10) | 0 | 0.6 (3.6) | |
| MALIGNANCY | |||||
| Malignancy (excluding NMSC) | 0 | 0 | 0 | 0 | |
| Lymphoma | 0 | 0 | 0 | 0 | |
| NMSC | 0 | 0 | 0 | 0 | |
| CARDIOVASCULAR EVENTS | |||||
| Adjudicated MACEa | 0 | 0 | 0 | 0 | |
| Adjudicated VTEb | 0 | 0 | 0.3 (1.8) | 0.1 (0.9) | |
| GASTROENTEROLOGICAL EVENTS | |||||
| Adjudicated gastrointestinal perforations* | 0 | 0.1 (0.7) | 0.3 (1.8) | 0 | |
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
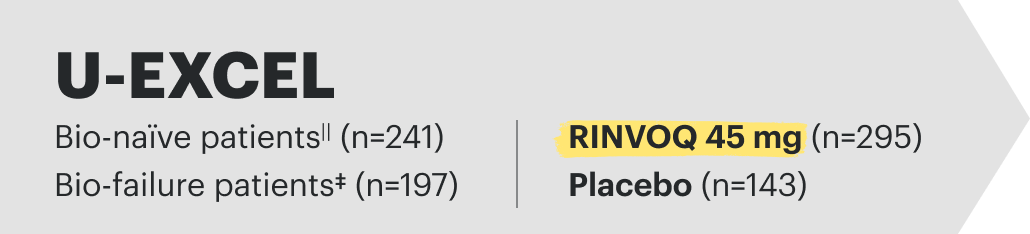
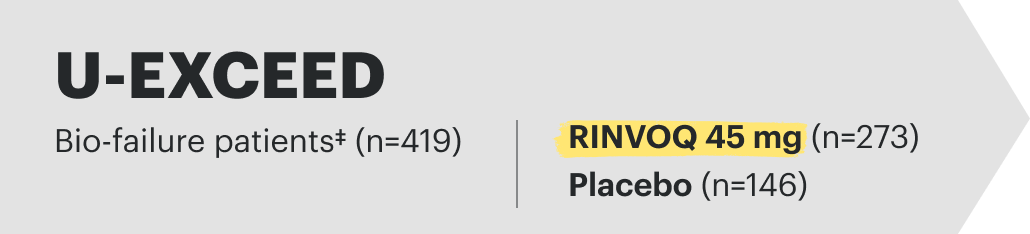
Induction studies for CD: (Phase 3 U-EXCEL and Phase3 U-EXCEED); integrated data inclusive of bio-failure† and bio-naïve‡ patients to represent placebo-controlled safety through 12 weeks for placebo (n=347) and RINVOQ (upadacitinib) 45 mg (n=674).9
Induction studies for UC: (Phase 2b U-ACHIEVE, Phase 3 U-ACHIEVE, and Phase 3 U-ACCOMPLISH); integrated to represent placebo-controlled safety through 8 weeks for placebo (n=378) and RINVOQ (upadacitinib) 45 mg (n=719).8
*In all Crohn's patients treated with RINVOQ 45 mg (N=938) during the induction studies, gastrointestinal perforations were reported in 4 patients (2 per 100 patient-years).
Safety Considerations
Consider the Benefits and Risks for the Individual Patient Prior to Initiating Therapy with RINVOQ
Common Adverse Events
Adverse reactions reported in ≥2% of patients1†
<<Swipe table to see more
| CROHN'S AT WEEK 52 | |||
|---|---|---|---|
| Placebo N=223 (%) | RINVOQ 15 mg QD N=221 (%) | RINVOQ 30 mg QD N=229 (%) | |
| ADVERSE REACTION | |||
| Upper respiratory tract infectiona | 11 | 14 | 12 |
| Pyrexia | 2 | 3 | 7 |
| Herpes zostera | 2 | 3 | 5 |
| Headachea | 1 | 3 | 5 |
| Acnea | 3 | 2 | 5 |
| Gastroenteritisa | 2 | 3 | 3 |
| Fatigue | 2 | 3 | 3 |
| Increased blood creatine phosphokinase | 1 | 2 | 3 |
| Elevated liver enzymesb | <1 | 2 | 3 |
| Leukopeniaa | <1 | 1 | 2 |
| Neutropeniaa | <1 | 1 | 2 |
| Bronchitisa | 0 | 1 | 2 |
| Pneumoniaa | 1 | 4 | 1 |
| Cough | 2 | 3 | 1 |
| UC AT WEEK 52 | |||
|---|---|---|---|
| Placebo N=245 (%) | RINVOQ 15 mg QD N=250 (%) | RINVOQ 30 mg QD N=251 (%) | |
| ADVERSE REACTION | |||
| Upper respiratory tract infectiona | 18 | 16 | 20 |
| Increased blood creatine phosphokinase | 2 | 6 | 8 |
| Neutropeniaa | 2 | 3 | 6 |
| Elevated liver enzymesb | 1 | 6 | 4 |
| Rasha | 4 | 5 | 5 |
| Herpes zoster | 0 | 4 | 4 |
| Folliculitis | 2 | 2 | 4 |
| Hypercholesterolemiaa | 1 | 2 | 4 |
| Influenza | 1 | 3 | 3 |
| Herpes simplexa | 1 | 2 | 3 |
| Lymphopeniaa | 2 | 3 | 2 |
| Hyperlipidemiaa | 0 | 2 | 2 |
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
Crohn's: Other adverse reactions reported in less than 2% of patients in the RINVOQ 15 and 30 mg group and at a higher rate than in the placebo group through Week 52 included hyperlipidemia, oral candidiasis, and hypercholesterolemia.
UC: Other adverse reactions reported in less than 2% of patients in the RINVOQ 45 mg group and at a higher rate than in the placebo group through Week 8 included herpes zoster and pneumonia.
aComposed of several similar terms.
bElevated liver enzymes composed of elevated ALT, AST, GGT, ALP, liver transaminases, hepatic enzymes, bilirubin, drug-induced liver injury and cholestasis.
Adverse reactions reported in ≥2% of patients1,*
<<Swipe table to see more
| CROHN'S AT WEEK 12 | |||
|---|---|---|---|
| Placebo (N=347) % | RINVOQ 45 mg QD (N=674) % | ||
| ADVERSE REACTION | |||
| Upper respiratory tract infectiona | 8 | 13 | |
| Anemiaa | 6 | 7 | |
| Acnea | 2 | 6 | |
| Pyrexia | 3 | 4 | |
| Increased blood creatine phosphokinase | 1 | 3 | |
| Influenza | 1 | 3 | |
| Herpes simplexa | 1 | 3 | |
| Leukopeniaa | 1 | 2 | |
| Neutropeniaa | <1 | 2 | |
| Herpes zoster | 0 | 2 | |
| UC AT WEEK 8 | |||
|---|---|---|---|
| Placebo (N=378) % | RINVOQ 45 mg QD (N=719) % |
||
| ADVERSE REACTION | |||
| Upper respiratory tract infectiona | 7 | 9 | |
| Acnea | 1 | 6 | |
| Increased blood creatine phosphokinase | 1 | 5 | |
| Neutropeniaa | <1 | 5 | |
| Elevated liver enzymesb | 2 | 3 | |
| Rasha | 1 | 4 | |
| Lymphopeniaa | 1 | 3 | |
| Folliculitis | 1 | 2 | |
| Herpes simplexa | <1 | 2 | |
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
CD: Other adverse reactions reported in less than 2% of patients in the RINVOQ 45 mg group and at a higher rate than in the placebo group through Week 12 included folliculitis, hypercholesterolemia, bronchitis, pneumonia, oral candidiasis, and hyperlipidemia.
UC: Other adverse reactions reported in less than 2% of patients in the RINVOQ 45 mg group and at a higher rate than in the placebo group through Week 8 included herpes zoster and pneumonia.
aComposed of several similar terms.
bElevated liver enzymes composed of elevated ALT, AST, GGT, ALP, liver transaminases, hepatic enzymes, bilirubin, drug-induced liver injury and cholestasis.
IR=intolerance or inadequate response; TNFi=tumor necrosis factor inhibitor.
Long-Term Exposure Data
Long-term exposure inclusive of ~2000 patient-years11
<<Swipe table to see more
| CROHN'S | ULCERATIVE COLITIS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 5212 | 5.1 YEARS MAX EXPOSURE13 | Week 5211 | 6.3 YEARS MAX EXPOSURE11 | ||||||||||
| ADVERSE EVENTS OF SPECIAL INTEREST (E/100 PYs unless otherwise noted) |
Placebo (N=223, PY=111.5) | RINVOQ 15 mg QD (N=221, PY=153.7) | RINVOQ 30 mg QD (N=229, PY=177.2) | RINVOQ 15 mg QD (N=221, PY=326.9) | RINVOQ 30 mg QD (N=229, PY=398.3) | Placebo (N=245, PY=135) | RINVOQ 15 mg QD (N=250, PY=199.4) | RINVOQ 30 mg QD (N=251, PY=218.5) | RINVOQ 15 mg QD (N=285, PY=622.7) | RINVOQ 30 mg QD (N=291, PY=721.9) | |||
| INFECTIONS | |||||||||||||
| Serious infections | 9.0 | 5.9 | 7.3 | 3.7 | 4.3 | 5.9 | 5.0 | 3.7 | 2.9 | 4.4 | |||
| Opportunistic infection (excluding TB, herpes zoster) | 0 | 0.7 | 0.6 | 0.6 | 0.3 | 1.5 | 1.0 | 0.9 | 0.3 | 0.6 | |||
| Active TB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Herpes zoster | 4.5 | 3.9 | 7.3 | 2.8 | 5.5 | 0 | 6.0 | 7.3 | 4.3 | 6.6 | |||
| MALIGNANCY¶ | |||||||||||||
| Malignancy (excluding NMSC) | 0 | 0.7 | 1.1 | 0.6 | 0.8 | 0.7 | 0.5 | 0.9 | 0.6 | 0.6 | |||
| Lymphoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| NMSC | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | 1.4 | 0 | 1.0 | |||
| CARDIOVASCULAR EVENTS¶ | |||||||||||||
| Adjudicated MACEa | 0 | 0 | 0 | 0 | 0 | 0.7 | 0 | 0.5 | 0 | 0.4 | |||
| Adjudicated VTEb | 0 | 0 | 0 | 0 | 0.3 | 0 | 1.0 | 0.9 | 0.5 | 0.6 | |||
| GASTROENTEROLOGICAL EVENTS¶ | |||||||||||||
| Adjudicated gastrointestinal perforations | 0.9 | 0.7 | 0.6 | 0.9 | 0.3 | 0.7 | 0 | 0 | 0 | 0 | |||
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
Week 52 UC Data: Patients responding to 8-week induction therapy with RINVOQ 45 mg randomized into the maintenance study. Data as of 08/2023.1,11
Long-Term Safety in UC: Patients from induction studies responding to RINVOQ 45 mg at Week 8 or 16 randomized into the maintenance study and additional time in the open-label extension study. Data as of 08/2023. UC maximum exposure: 15 mg ~6.3 years / 30 mg ~5.3 years. UC median exposure: 15 mg ~1.6 years / 30 mg ~3.0 years.
Week 52 CD Data: Patients responding to 12-week induction therapy with RINVOQ 45 mg randomized into the maintenance study. Data as of 1/2023.1,6
Long-Term Safety in CD: Patients from induction studies responding to RINVOQ 45 mg at Week 12 randomized into the maintenance study and additional time in the open-label extension study. Data as of 08/2023. CD maximum exposures: 15 mg ~5.1 years / 30 mg ~5.3 years, CD median exposure: 15 mg ~1.0 year / 30 mg ~1.8 years.
<<Swipe table to see more
| Week 522 | 6.3 MAX EXPOSURE2 | ||||
|---|---|---|---|---|---|
| ADVERSE EVENTS OF SPECIAL INTEREST (E/100 PYs unless otherwise noted) |
Placebo n=245 (%) PYs=135 | RINVOQ 15 mg QD n=250 (%) PYs=199.4 | RINVOQ 30 mg QD n=251 (%) PYs=218.5 | RINVOQ 15 mg QD n=285 (%) PYs=622.7 | RINVOQ 30 mg QD n=291 (%) PYs=721.9 |
| INFECTIONS | |||||
| Serious infections | 5.9 | 5.0 | 3.7 | 2.9 | 4.4 |
| Opportunistic Infection (excluding TB, herpes zoster) | 1.5 | 1.0 | 0.9 | 0.3 | 0.6 |
| Active TB | 0 | 0 | 0 | 0 | 0 |
| Herpes Zoster | 0 | 6.0 | 7.3 | 4.3 | 6.6 |
| MALIGNANCY | |||||
| Malignancy (excluding NMSC) | 0.7 | 0.5 | 0.9 | 0.6 | 0.6 |
| Lymphoma | 0 | 0 | 0 | 0 | 0 |
| NMSC | 0 | 0 | 1.4 | 0 | 1.0 |
| CARDIOVASCULAR EVENTS | |||||
| Adjudicated MACEa | 0.7 | 0 | 0.5 | 0 | 0.4 |
| Adjudicated VTEb | 0 | 1.0 | 0.9 | 0.5 | 0.6 |
| GASTROENTEROLOGICAL EVENTS | |||||
| Adjudicated gastrointestinal perforations | 0.7 | 0 | 0 | 0 | 0 |
Week 52 Safety: Patients responding to 8 week induction therapy with RINVOQ 45 mg randomized into the maintenance study. Data as of 08/2022.
Long-term Safety: Patients from induction studies responding to RINVOQ 45 mg at Week 8 or 16 randomized into the maintenance study and additional time in the open label extension study. Data as of 08/2023. UC maximum exposure: 15 mg - 6.3 years/5.3 years. UC median exposure: 15 mg - 1.6 years / 30 mg - 3 years.
Adverse Reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
<<Swipe table to see more




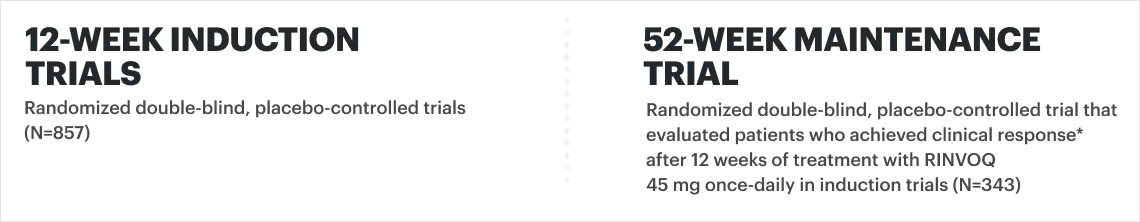




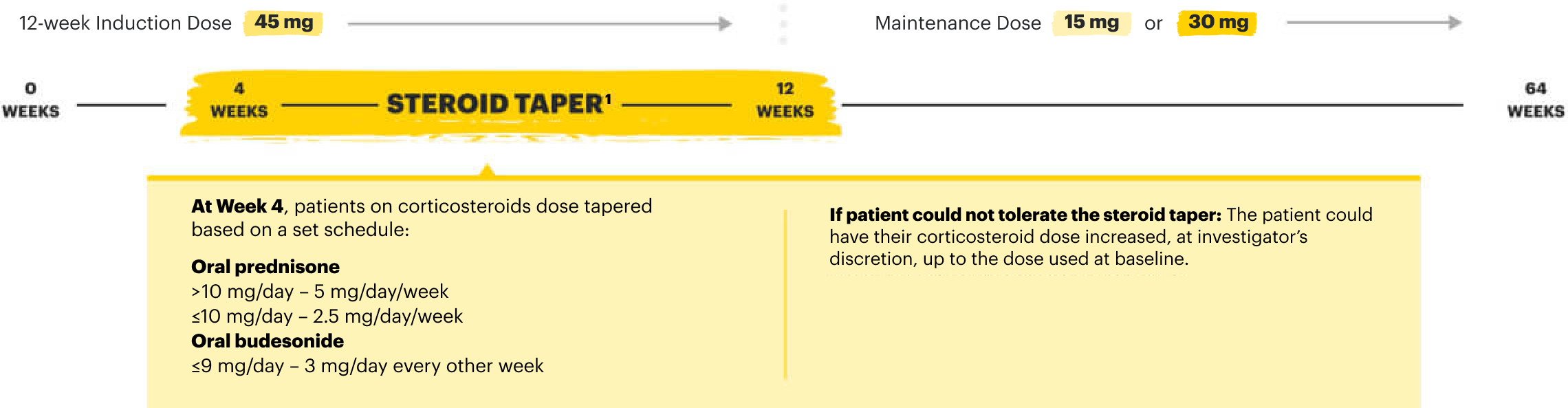





![52-week maintenance trial (randomized double-blind, placebo-controlled trial that evaluated patients who achieved clinical response after 12 weeks of treatment with RINVOQ 45 mg once-daily in induction trials [N=343]).](/content/dam/rinvoqhcpivy/images/gastroenterology/cd/maintenance-trials-mobile.png)