RINVOQ vs
DUPIXENT® (dupilumab)
LEVEL UP Study
Explore head-to-head data for RINVOQ vs DUPIXENT and switch data for RINVOQ among DUPIXENT inadequate responders (<EASI 75).
US-MULT-250253
WARNING: Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis
INDICATION
RINVOQ is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate to severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies are inadvisable.
Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
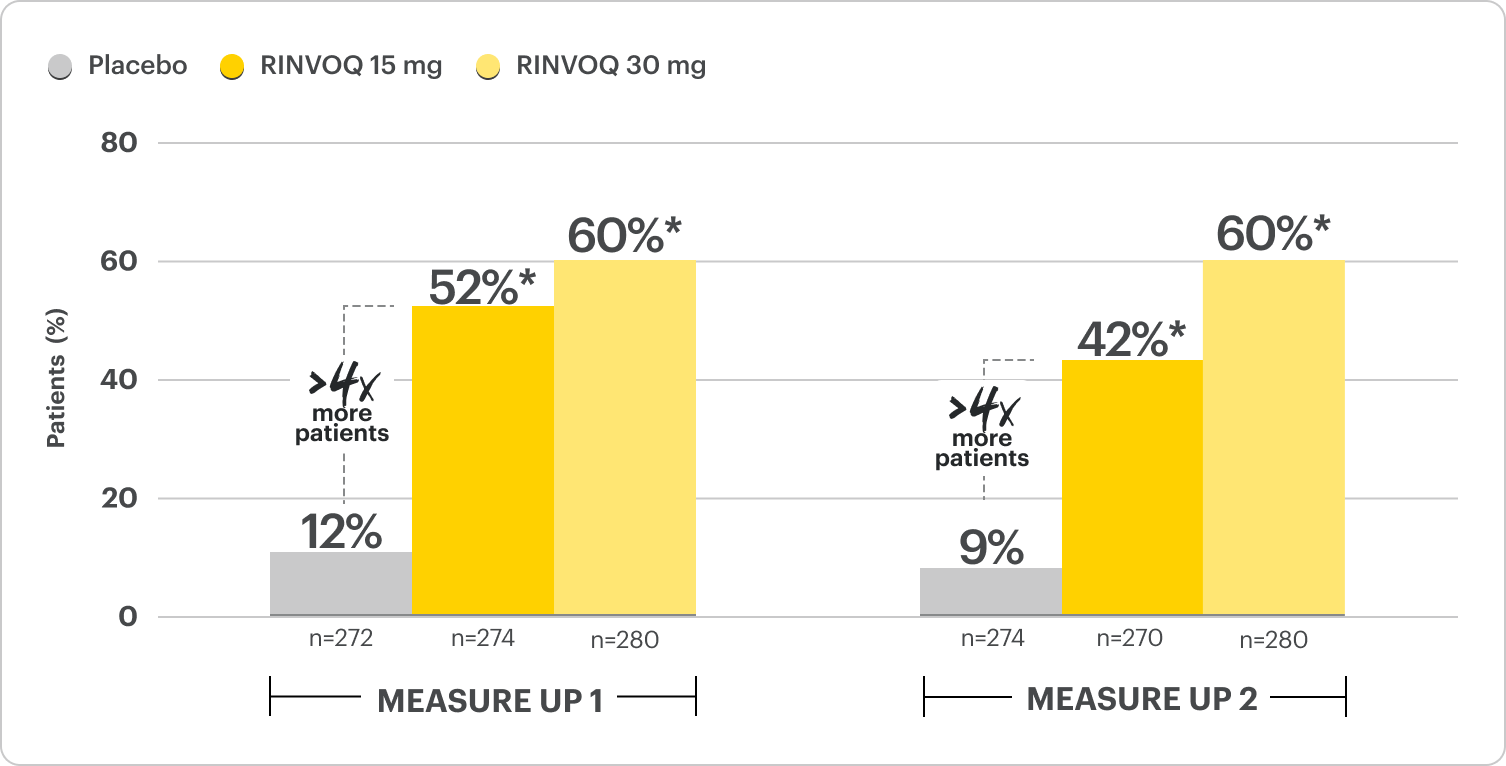
Rapid Itch Relief in 4x More Patients vs Placebo at Week 16
Proportion of patients with improvement in WP-NRS ≥4 at Week 161,2
Monotherapy Results; Ranked Secondary Endpoint
RECOMMENDED DOSAGE IN AD1
PROPORTION OF PATIENTS ACHIEVING CO-PRIMARY ENDPOINTS AT WEEK 161,2
EASI 75 at Week 16
MEASURE UP 1
70%* (RINVOQ 15 mg, n=281)
80%* (RINVOQ 30 mg, n=285)
16% (placebo, n=281)
MEASURE UP 2
60%* (RINVOQ 15 mg, n=276)
73%* (RINVOQ 30 mg, n=282)
13% (placebo, n=278)
vIGA 0/1 at Week 16
MEASURE UP 1
48%* (RINVOQ 15 mg, n=281)
62%* (RINVOQ 30 mg, n=285)
8% (placebo, n=281)
MEASURE UP 2
39%* (RINVOQ 15 mg, n=276)
52%* (RINVOQ 30 mg, n=282)
5% (placebo, n=278)
Proportion of patients with WP-NRS ≥4 response as early as 2 days after first dose2
Ranked secondary endpoint
MEASURE UP 1
16%* RINVOQ 15 mg
3% Placebo
MEASURE UP 2
12%* RINVOQ 15 mg
3% Placebo
Proportion of patients with improvement in WP-NRS ≥4 at Week 12
Ranked secondary endpoint
MEASURE UP 1
15%* RINVOQ 15 mg
20%* RINVOQ 30 mg
0% Placebo
MEASURE UP 2
7%* RINVOQ 15 mg
16%* RINVOQ 30 mg
1% Placebo
*P≤0.001; RINVOQ vs placebo. NRI-C; ITT.2
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
Little to No Itch (WP-NRS 0/1) Rates Observed at Week 16 and ~4 Years
Proportion of patients achieving WP-NRS 0/1 at Week 16 and at ~4 years3,4
Integrated Monotherapy Results From MEASURE UP 1 and 2; All Data Are Observed Cases*
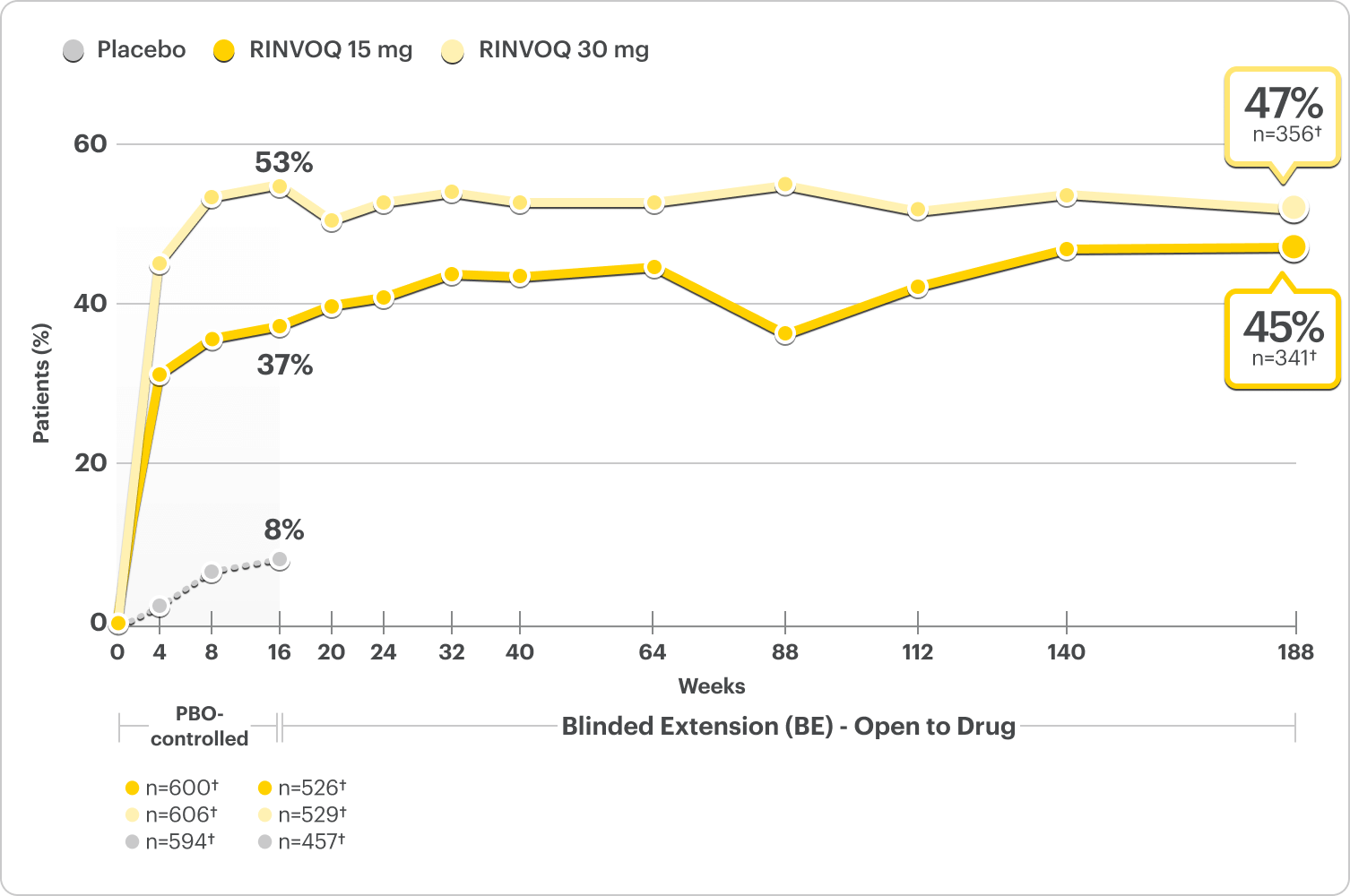
DATA LIMITATIONS: WP-NRS 0/1 data at Week 16 and at ~4 years were prespecified, non-ranked endpoints and not controlled for multiplicity; thus, results cannot be considered statistically significant.
BE LIMITATIONS: There is potential for enrichment of BE data; awareness of active treatment may cause bias related to overall treatment effect. Topicals, if used, were no longer considered rescue. No statistical or clinical conclusions can be drawn.4
WP-NRS 0/1 captured daily through Week 16 and averaged for prior week.4
*A sub-analysis of the MEASURE UP blinded extension data including only patients who were originally randomized to RINVOQ, completed the RCT, and enrolled in the blinded extension.4
†n-value includes patients whose baseline WP-NRS is >1.
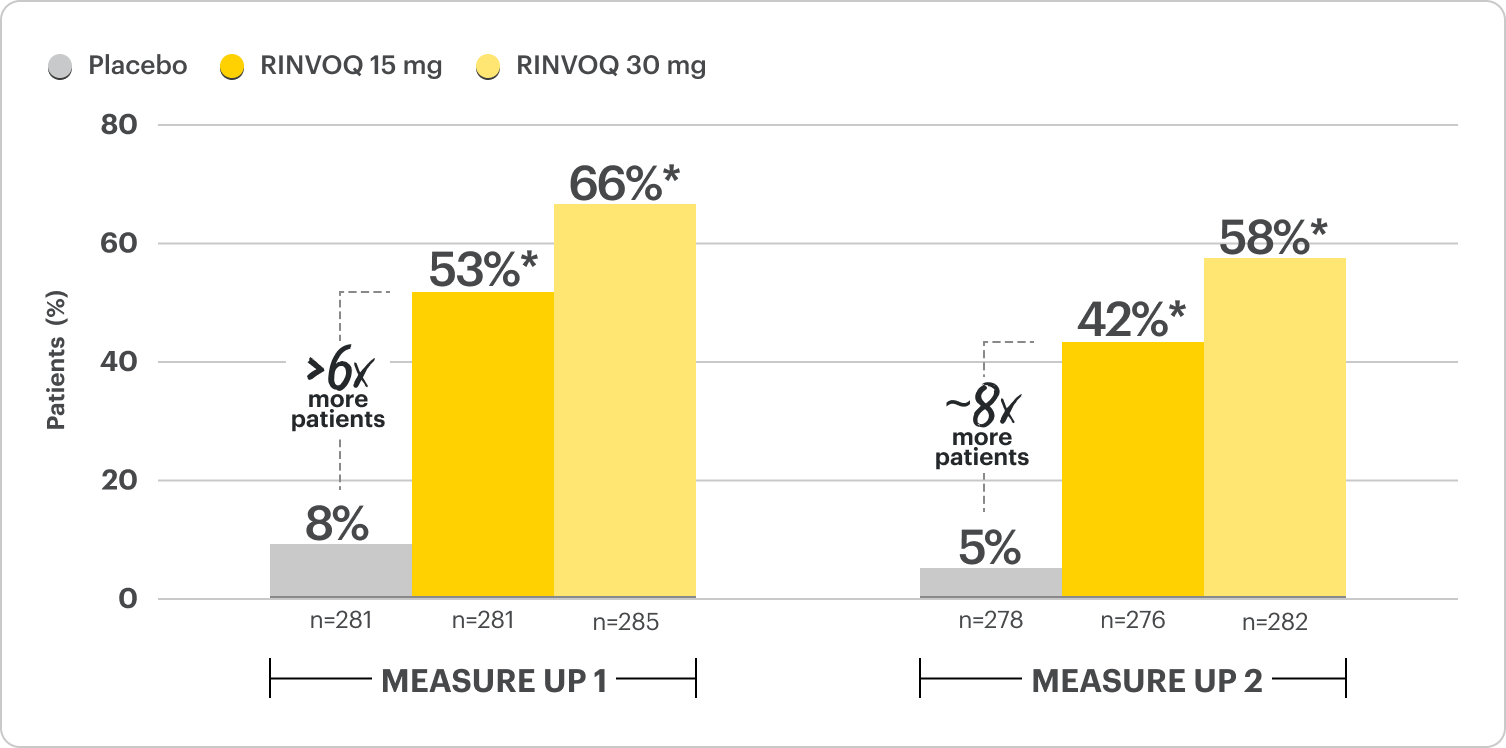
Rapid Skin Clearance in 4-7x More Patients vs Placebo at Week 16
Proportion of patients achieving EASI 75 and vIGA 0/1 at Week 161,2
Monotherapy Results; Co-Primary Endpoints
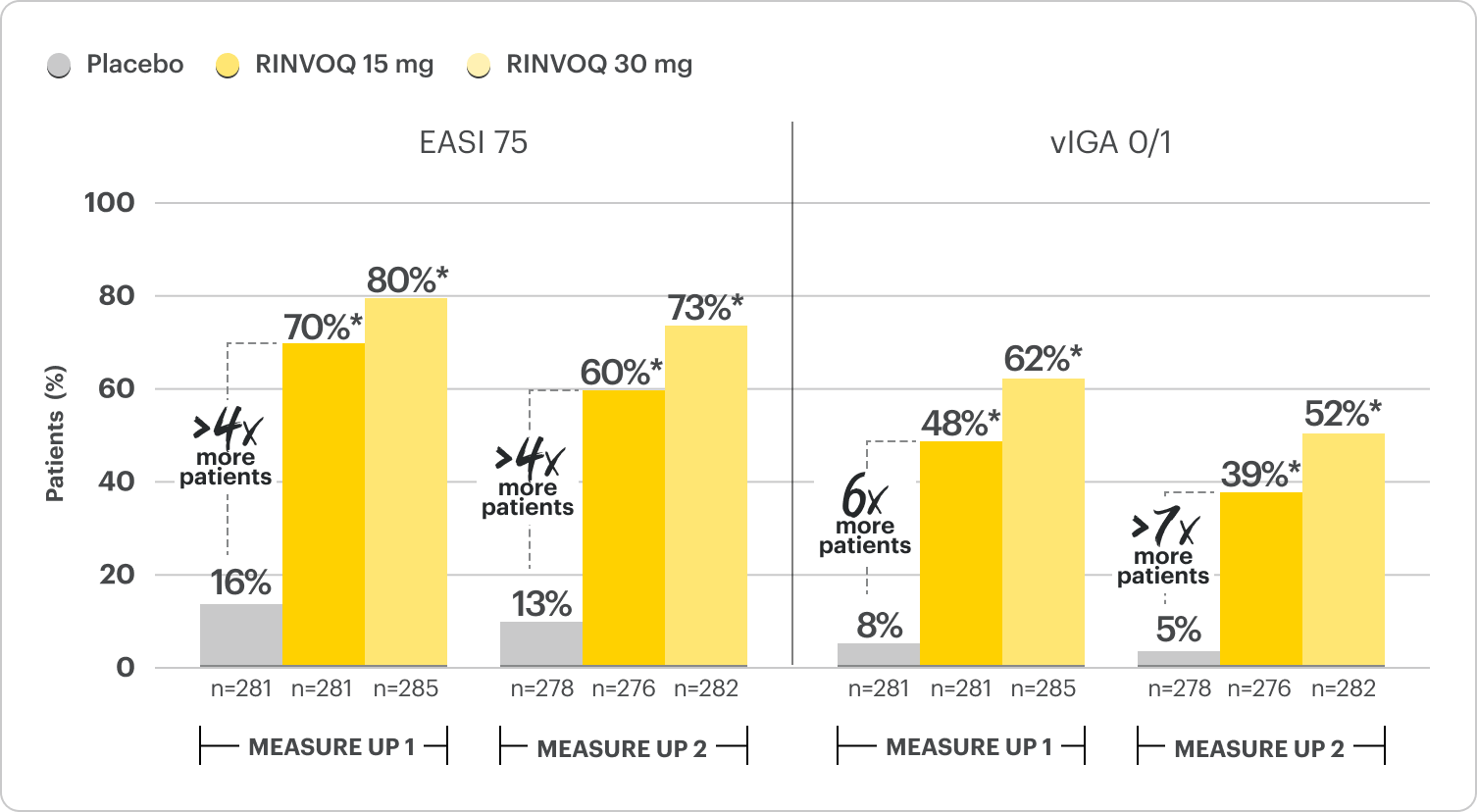
*P≤0.001; RINVOQ vs placebo. NRI-C; ITT.
RECOMMENDED DOSAGE IN AD1
Proportion of patients achieving EASI 75 response observed as early as 2 weeks2
Ranked secondary endpoint
MEASURE UP 1
38%* RINVOQ 15 mg
47%* RINVOQ 30 mg
4% Placebo
MEASURE UP 2
33%* RINVOQ 15 mg
44%* RINVOQ 30 mg
4% Placebo
Serious Infections: RINVOQ-treated patients are at increased risk of serious bacterial (including tuberculosis [TB]), fungal, viral, and opportunistic infections leading to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids.
Mortality: A higher rate of all-cause mortality, including sudden cardiovascular (CV) death, was observed with a Janus kinase inhibitor (JAKi) in a study comparing another JAKi with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients ≥50 years with ≥1 CV risk factor.
Malignancies: Malignancies have occurred in RINVOQ-treated patients. A higher rate of lymphomas and lung cancer (in current or past smokers) was observed with another JAKi when compared with TNF blockers in RA patients.
Major Adverse Cardiovascular Events: A higher rate of CV death, myocardial infarction, and stroke was observed with a JAKi in a study comparing another JAKi with TNF blockers in RA patients ≥50 years with ≥1 CV risk factor. History of smoking increases risk.
Thromboses: Deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated for inflammatory conditions with JAK inhibitors, including RINVOQ. A higher rate of thrombosis was observed with another JAKi when compared with TNF blockers in RA patients.
Hypersensitivity: RINVOQ is contraindicated in patients with hypersensitivity to RINVOQ or its excipients.
Other Serious Adverse Reactions: Hypersensitivity Reactions, Gastrointestinal Perforations, Laboratory Abnormalities, and Embryo-Fetal Toxicity.
Robust 90% Skin Clearance Achieved at Week 16
Proportion of patients achieving EASI 90 skin clearance at Week 161,2*
Monotherapy Results; Ranked Secondary Endpoint
*P≤0.001; RINVOQ vs placebo. NRI-C; ITT.
Durable Rates of 90% Skin Clearance Observed at ~4 Years
Proportion of patients achieving EASI 90 at ~4 years3,4
Integrated Monotherapy Results From MEASURE UP 1 and 2. All Data Are Observed Cases*
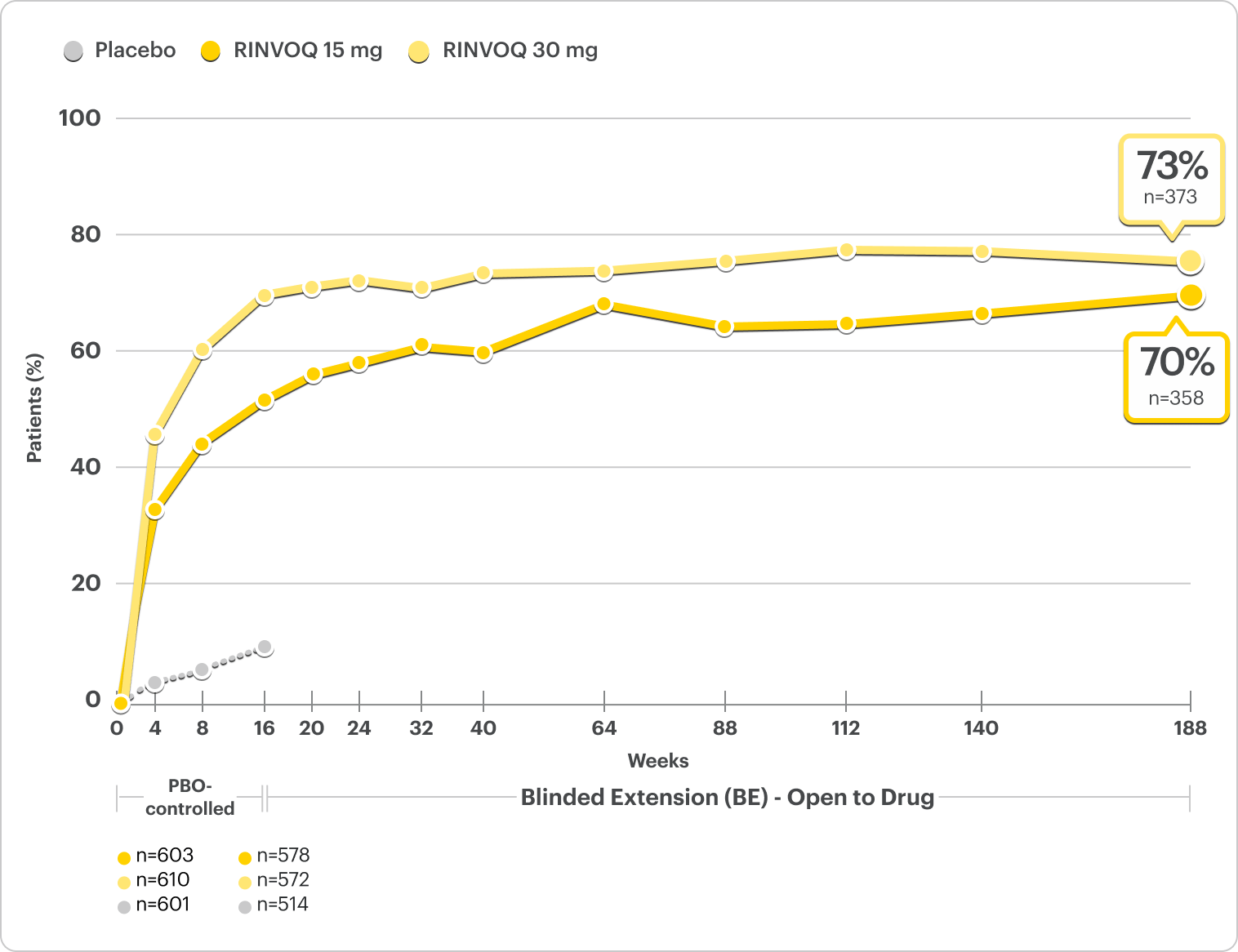
DATA LIMITATIONS: Data at ~4 years were prespecified, non-ranked endpoints, not controlled for multiplicity; thus, results cannot be considered statistically significant.
BE LIMITATIONS: There is potential for enrichment of BE data; awareness of active treatment may cause bias related to overall treatment effect. Topicals, if used, were no longer considered rescue. No statistical or clinical conclusions can be drawn.
*A sub-analysis of the MEASURE UP blinded extension data including only patients who were originally randomized to RINVOQ, completed the RCT, and enrolled in the blinded-extension.
100% Skin Clearance Rates Observed at ~4 Years
Proportion of patients achieving EASI 100 at ~4 years3,4*
Integrated Monotherapy Results From MEASURE UP 1 and 2; All Data Are Observed Cases†
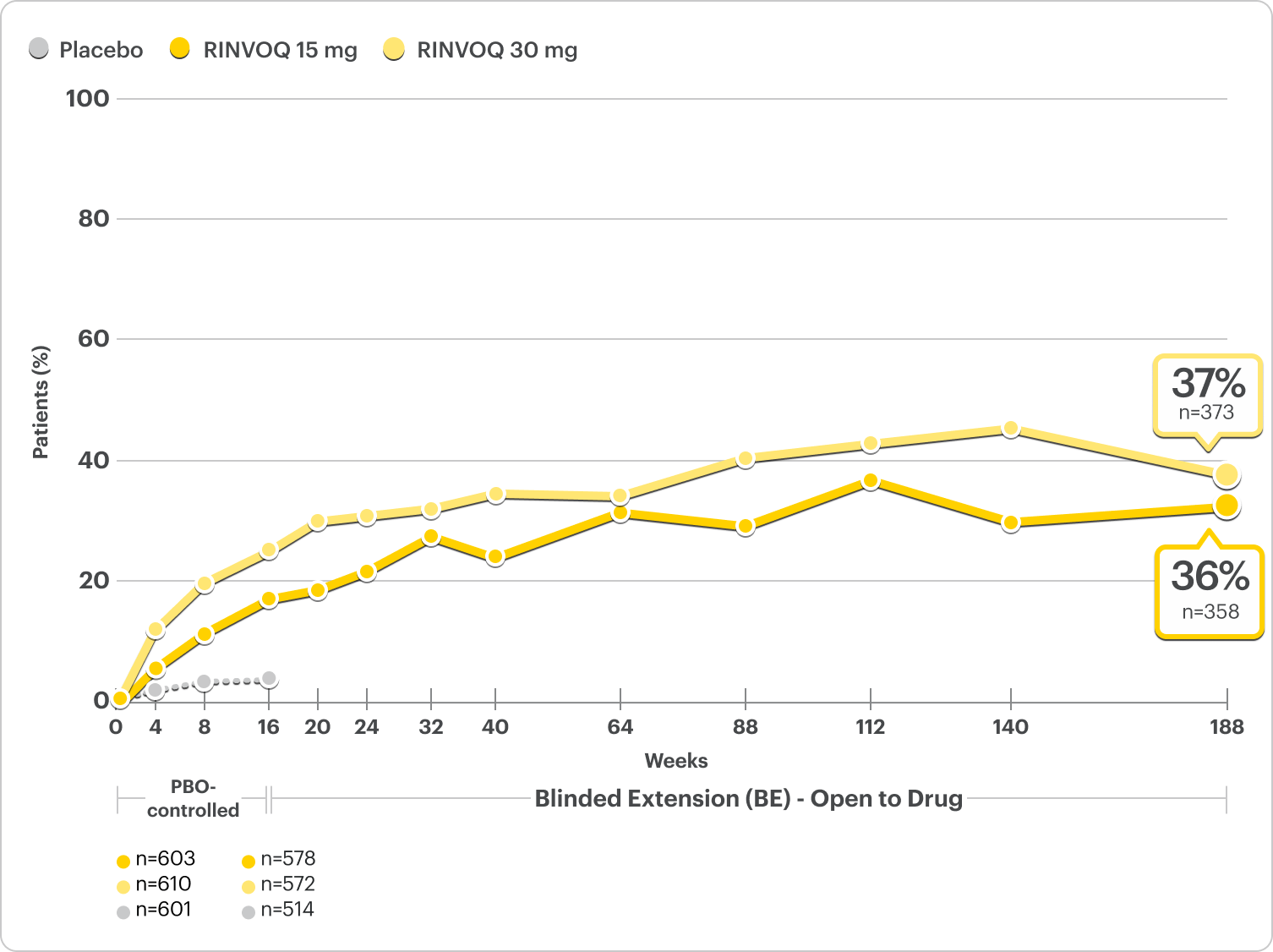
Robust EASI 100
Responses at Week 16
Ranked secondary endpoint1,2
MEASURE UP 1
17%* RINVOQ 15 mg (n=281)
27%* RINVOQ 30 mg (n=285)
2% Placebo (n=281)
MEASURE UP 2
14%* RINVOQ 15 mg (n=276)
19%* RINVOQ 30 mg (n=282)
1% Placebo (n=278)
*P≤0.001; RINVOQ vs placebo. NRI-C; ITT.
†A sub-analysis of the MEASURE UP blinded extension data including only patients who were originally randomized to RINVOQ, completed the RCT, and enrolled in the blinded extension.
DATA LIMITATIONS: Data at ~4 years were prespecified, non-ranked endpoints, not controlled for multiplicity; thus, results cannot be considered statistically significant.
BE LIMITATIONS: There is potential for enrichment of BE data; awareness of active treatment may cause bias related to overall treatment effect. Topicals, if used, were no longer considered rescue. No statistical or clinical conclusions can be drawn.
Please see Important Safety Information, including BOXED WARNING on Serious Infections, Mortality, Malignancies, Major Adverse Cardiovascular Events, and Thrombosis, below.
Patients on RINVOQ achieved an improvement in itch as measured by a ≥4-point reduction in WP-NRS at Week 16 in an integrated analysis of MEASURE UP 1 and 2
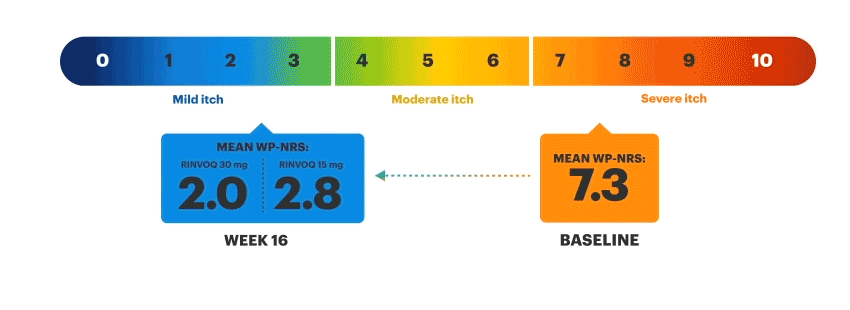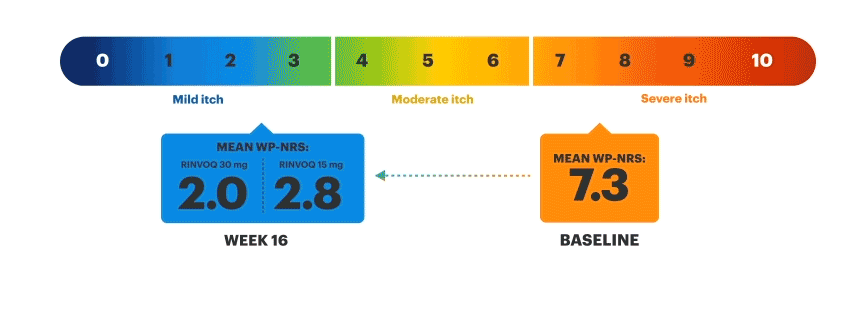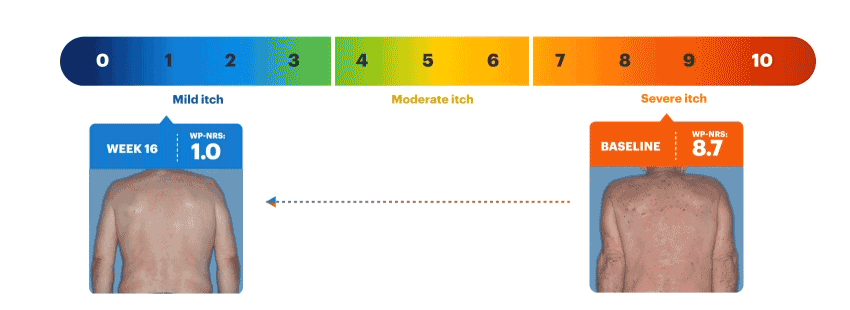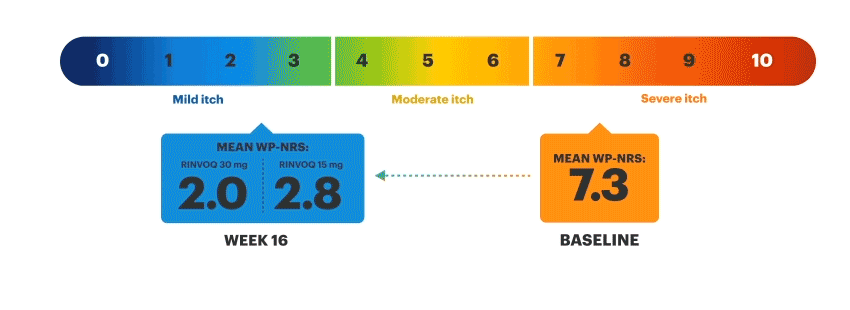
Actual MEASURE UP patient treated with RINVOQ in a clinical trial. The typical RINVOQ patient achieved a 4-point improvement in the WP-NRS score from baseline. Individual results may vary. The Worst Pruritus Numerical Rating Scale (WP-NRS) is a validated tool for measurement of itch severity.6
Inclusion criteria for RINVOQ phase 3 clinical trials included active moderate to severe atopic dermatitis, defined by EASI ≥16, vIGA ≥3, BSA ≥10%, and WP-NRS ≥4. A patient with baseline score of 7 would need a score of 3 or lower to achieve improvement in WP-NRS ≥4.
Visible Results at Week 161,2,8,9
EASI=Eczema Area and Severity Index; EASI 75=improvement of at least 75% in lesion extent and severity; EASI 90=improvement of at least 90% in lesion extent and severity; WP-NRS=Worst Pruritus Numerical Rating Scale; WP-NRS 0/1=little to no itch on the WP-NRS (for patients with WP-NRS >1 at baseline); Improvement in WP-NRS ≥4=improvement (reduction) in WP-NRS ≥4 points from baseline in patients with WP-NRS >4 at baseline.1,4
RINVOQ is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate to severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies are inadvisable.
Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
REFERENCES:
RINVOQ vs
DUPIXENT® (dupilumab)
LEVEL UP Study
Explore head-to-head data for RINVOQ vs DUPIXENT and switch data for RINVOQ among DUPIXENT inadequate responders (<EASI 75).