PODCAST TRANSCRIPT
Narrator: At the intersection of science and clinical experience in IBD, this is Gut Reactions. Settle in, as our host, Dr. David Rubin, brings leading gastroenterologists together for one-on-one conversations. This podcast is brought to you by AbbVie.
Dr. David Rubin: Welcome to the Gut Reactions podcast. I'm your host, Dr. David Rubin, Chief of the Section of Gastroenterology, Hepatology and Nutrition and Director of the Inflammatory Bowel Disease Center at the University of Chicago. Today, I'll be joined by Dr. Oriana Damas to discuss objective measures in ulcerative colitis, mucosal healing, and how it can impact the lives of our patients who have moderate to severe ulcerative colitis. We'll also talk about a treatment option that could help your patients achieve mucosal healing.
Oriana Damas is an MD and has her master’s degree in Clinical and Translational Investigation. She's an Associate Professor of Medicine at the University of Miami, where she is also an Inflammatory Bowel Disease Physician Scientist and Director of Translational Studies for their Crohn's and Colitis Center. Her research is focused on understanding environmental and dietary patterns that can influence disease risk and modify IBD outcomes. Her current Career Development Award from the NIH focuses on understanding dietary, genetic, and microbiome factors that lead to gut inflammation in Hispanic patients, which represent an emerging population of IBD.
Oriana, I’m so happy to have you here on Gut Reactions.
Dr. Oriana Damas: Thank you, David. It's a pleasure to be here and I'm excited for our conversation.
Dr. David Rubin: Many of our listeners may be familiar with the STRIDE-II recommendations, but to set the stage for our topics today, let's begin with a brief overview.
Before STRIDE-II, we had the initial STRIDE recommendations, which were published in 2015 and formalized the idea of treating to a target in inflammatory bowel disease. The original STRIDE recommendations on treatment targets included patient-reported outcomes, such as rectal bleeding and stool frequency, as well as objective measures of disease control with a focus on endoscopy. Since STRIDE launched, new data came out that led to the STRIDE-II.
STRIDE-II recommends short-term, intermediate, and long-term targets in the treatment of IBD. As the timeframe for reaching these targets increases, the measures become increasingly objective. As a member of the International Organization for the Study of IBD committee that was responsible for drafting these recommendations, a key insight that led to STRIDE-II was the desire to achieve long-term outcomes for our patients. Oriana, would you share with our listeners the STRIDE-II targets and how they align with your real-world management of IBD patients? For this episode of the podcast, we’ll keep the conversation focused on moderate to severe ulcerative colitis.
Dr. Oriana Damas: Sure. So, I think it's important to recognize that why were the STRIDE-II targets developed in the first place? I think there's increased recognition that we shouldn't just focus on symptom improvement. We should also look for more significant metrics that really correlate with improved outcomes for patients. And those are both biomarker improvement and, in the long term, endoscopic and maybe even histologic improvement.
And so, that's really why the STRIDE-II criteria were developed in the first place. As a short-term target, the STRIDE-II signals symptomatic response as being the first short-term target for providers and for patients. And what does that mean? Specifically, it means a decrease in rectal bleeding for ulcerative colitis, as well as a decrease in the stool frequency, followed by actual symptomatic remission, which generally means no blood in the stool and normal stool frequency.
As an intermediate target, then STRIDE-II recommends the biomarker measures. It's that fecal calprotectin and the C-reactive protein in the blood. We want to normalize both of those metrics and we want to assess that as an intermediate target in between symptomatic improvement, which is a short-term target, and the long-term target, which is endoscopic slash histologic improvement.
So, in my clinical experience, biomarkers can be helpful measures to assess underlying inflammation and also to assess whether your patient is responding to that therapy in an objective measure. So, I would normally recommend completing biomarker evaluation in parallel with your clinical follow-up. So that really depends on whether your patients are really symptomatic when you first meet them or whether they're somewhat controlled on steroids and you're starting them on that medication.
So, I use the level of acuity to decide when I bring those patients back. So typically, if they're very active and I'm concerned that I need this drug to really start acting right away, if not, they may end up in the hospital, for example, I try to have them seen much sooner in our follow-up appointments, typically at 6- to 8-week mark.
And I also check a fecal calprotectin, again, that measure of biomarker activity, which is an intermediate target for STRIDE-II. And I'll typically check that around six to eight weeks as well.
If I find that patients are relatively stable, then I'll schedule a follow-up appointment at around a 12-week mark. In that moment, I will also ask patients to do a stool sample typically a week or so before their appointment at 12 weeks in order to assess what their fecal calprotectin is at that point in time. Much later on, if I find that there is in fact both clinical, right, that short-term target, and intermediate target improvements with fecal calprotectin, then I'm reassured and I'll have the patient come back at around five months or so.
Dr. David Rubin: Oriana, can you share with our listeners the long-term targets identified in STRIDE-II and your clinical experience with them?
Dr. Oriana Damas: So, for long-term targets, STRIDE-II identifies endoscopic healing as a preferred long-term treatment target in IBD. In ulcerative colitis, endoscopic healing is generally defined as achieving a Mayo endoscopy subscore of 0 or 1, which is normal or mild disease. The STRIDE-II recommendations note how a score of 0 is associated with superior disease outcomes. And the end goal, right, the long-term target as per STRIDE-II criteria and what I follow in my clinical practice, is that if patients are improving both symptomatically and biochemically, I'll plan to do a colonoscopy at around 8- to 12-months to really hit that target, which is what we know has the best outcomes possible, which is endoscopic healing. This gives me visible evidence of disease control, and this is what is supported in all the studies looking at treatment outcomes.
Endoscopic healing is a critical treatment goal for my UC patients. In my practice, around 8 to 12 months depending on disease severity, but no later than typically a year after initiating therapy, I'll have the patient back for a colonoscopy where I look for improvement of the appearance of the mucosa. When I see a patient improve to normal or mild disease, this gives me visible evidence of disease control.
And I think that's where the STRIDE-II criteria really add value to clinical practice because they tell us, don't just check clinical symptoms, make sure that you are also achieving biochemical and endoscopic healing as well.
Dr. David Rubin: Yeah, I say to patients, I want you to feel better right away because that'll improve your quality of life. But if we want it to last, we want to look for objective measures that we're controlling the disease process. So feeling better means decreased stool frequency, elimination of bleeding, of course, but also elimination of urgency.
And then we start talking about how we would assess whether the disease is under control, looking at labs and, of course, endoscopic improvement as well. So, I think that this makes good sense, but it's important to make sure your patients know where you're going and that we're on the same page.
Building on our conversation of long-term UC treatment targets, let's dive deeper into the topic of mucosal healing. Over recent years, the definition of mucosal healing in ulcerative colitis has evolved to include both endoscopic and histologic results. In fact, STRIDE-II includes histologic activity as an aspirational therapeutic target in UC. When used in conjunction with endoscopic healing, it can represent a deeper level of healing. Evidence suggests that achieving histological remission in conjunction with endoscopic healing is associated with improved patient outcomes such as long-term remission, a reduced need for surgery, and a lower risk of cancer.
Before we go further, I think we do need to acknowledge that there are multiple indices used to assess histologic activity, and there's not currently one standard definition of histologic remission. Oriana, how are you incorporating histology into your practice to evaluate disease control, if you're doing it at all?
Dr. Oriana Damas: In addition to endoscopy, which is a key part of ongoing management, I commonly perform biopsies to assess for histologic assessment again around that 8- to 12-month mark. And this will be read by our central lab or GI pathologist. So, in my practice, I have found that it can be a helpful predictor of future relapse. I typically take biopsies at different segments, as it provides a roadmap for disease activity. Often there is a parallel and correlation between endoscopic outcomes and histologic findings. I'll tell you that, histologic activity, we still don't have very clear data on what to do with it as far as the research studies and the treatment targets. When I have found it to be particularly helpful is when I grade an endoscopic score as a Mayo 1 and it ends up being a little bit more severe disease on histology. In that case, I may consider doing a different management option based on the biopsy results.
Dr. David Rubin: I do the same. Are you changing treatments if the patient doesn't achieve histologic remission, though?
Dr. Oriana Damas: I wouldn't change treatment just based on achievement or lack of achievement, I should say, of histologic remission, because our data does not support that yet. And so, you know, I think if a patient has a Mayo score of 0 or a Mayo score of 1, feels great, has completely normal laboratory values, including biochemical markers of inflammation, but on the biopsies, there's still some mildly active disease, I really do not think that at this point, we should be trying to be more aggressive with the treatment pattern for patients if, in my mind, they've already achieved endoscopic improvement and a good outcome overall.
Dr. David Rubin: In my practice, I take a similar approach with using it as more of a holistic component of assessing disease control in conjunction with an endoscopy. In the STRIDE-II recommendations, histological healing is not yet a formal target for a few reasons, including the difficulty to achieve it, a lack of consensus around what a standard measurement index should be, and of course, cost reasons. That said, it's interesting to see that recent clinical trials are incorporating composite endoscopic and histologic endpoints as secondary endpoints. The clinical trials may use different names and criteria for their endpoints, such as histologic endoscopic mucosal improvement or endoscopic histologic mucosal improvement, so it's important for us to understand what a trial's endpoint is measuring so we can appropriately interpret the result.
So, Oriana, how do you consider histologic and endoscopic outcomes when you're evaluating the different treatments for UC?
Dr. Oriana Damas: So, when evaluating ulcerative colitis therapies, objective evidence can be an important component of my holistic treatment decision. So for every patient, I'm weighing the risks, the benefits of the treatment, their treatment history, and their current presentation. So when evaluating clinical trial results, endoscopic results are important. I need to know the drug can work beyond symptom control to really address underlying inflammation.
Stringent endpoints that include histology, like histoendoscopic mucosal improvement, are also valuable. And while I'm not using histologic evidence on its own to make a treatment switch decision, as we mentioned earlier, a drug achieving stringent endpoints within its clinical trials can give me increased confidence on the therapy's long-term efficacy profile.
Dr. David Rubin: Now that we've discussed the goals of UC treatment, we'll be discussing RINVOQ, or upadacitinib, and its role in treating adult patients with moderate to severe UC who have had an inadequate response or intolerance to one or more TNF blockers. Let's take a moment to review some important context for RINVOQ before discussing the clinical data.
RINVOQ is a once-daily oral Janus kinase inhibitor indicated for the treatment of adults with moderately to severely active ulcerative colitis who have had an inadequate response or intolerance to one or more tumor necrosis factor blockers. RINVOQ is not recommended for use in combination with other JAK inhibitors, biological therapies for ulcerative colitis, or with potent immunosuppressants.
RINVOQ is contraindicated in patients with a hypersensitivity to RINVOQ or its excipients.
Patients treated with RINVOQ are at an increased risk of serious infections that can lead to hospitalization or death. Most of these infections occurred in patients who were concurrently taking immunosuppressants.
A number of safety findings were observed in a study comparing another JAK inhibitor against TNF blockers in rheumatoid arthritis patients who were 50 and older and had one or more cardiovascular risk factors. A higher rate of all-cause mortality, thrombosis, lymphomas, and lung cancer was observed with the other JAK inhibitor. Furthermore, the JAK inhibitor group demonstrated a higher rate of cardiovascular death, myocardial infarction, and stroke. There is increased risk for patients with a history of smoking.
Malignancies have also been reported in patients treated with RINVOQ. Patients treated with JAK inhibitors for inflammatory conditions have also experienced deep venous thrombosis, pulmonary embolism, and arterial thrombosis.
Other serious adverse reactions associated with RINVOQ include hypersensitivity reactions, gastrointestinal perforations, laboratory abnormalities, and embryo-fetal toxicity.
Please continue listening and stay tuned for additional Important Safety Information within this podcast.
Dr. David Rubin: Turning our attention to RINVOQ, this is a treatment that you and I both have experience prescribing, going back to the clinical trials.
RINVOQ had two, 8-week, multicenter, double-blind, placebo-controlled UC induction trials. Participants on RINVOQ who achieved clinical response at Week 8 with 45 mg of RINVOQ were moved into the U-ACHIEVE maintenance trial.
The clinical trials had a primary endpoint of clinical remission—defined as no rectal bleeding, reduced stool frequency, and an endoscopic subscore of 1 or less without friability—and ranked secondary endpoints, which included clinical response per an adapted Mayo score, endoscopic improvement, and histologic-endoscopic mucosal improvement.
Oriana, tell us how RINVOQ ultimately did against its primary endpoints.
Dr. Oriana Damas: RINVOQ achieved all primary and secondary endpoints. RINVOQ met its primary endpoints of clinical remission at Week 8 in the induction trials, delivering powerful rates compared to placebo. In the 52-week maintenance trial, RINVOQ met its primary endpoint, demonstrating durable remission at Week 52. 42% of the 148 patients on RINVOQ 15 mg, and 52% of the 154 patients on RINVOQ 30 mg, achieved clinical remission, compared to 12% of the 149 patients on placebo.
Dr. David Rubin: In the U-ACHIEVE maintenance trial, 49% of the 148 patients on RINVOQ 15 mg and 62% of the 154 patients on RINVOQ 30 mg achieved endoscopic improvement, compared with the 14% of the 149 patients receiving placebo.
What’s important to consider is where the patients started at the entrance of the induction studies—approximately 70% of patients began as Mayo endoscopic subscore of 3 or severe disease, and 30% as a Mayo endoscopic subscore of 2.
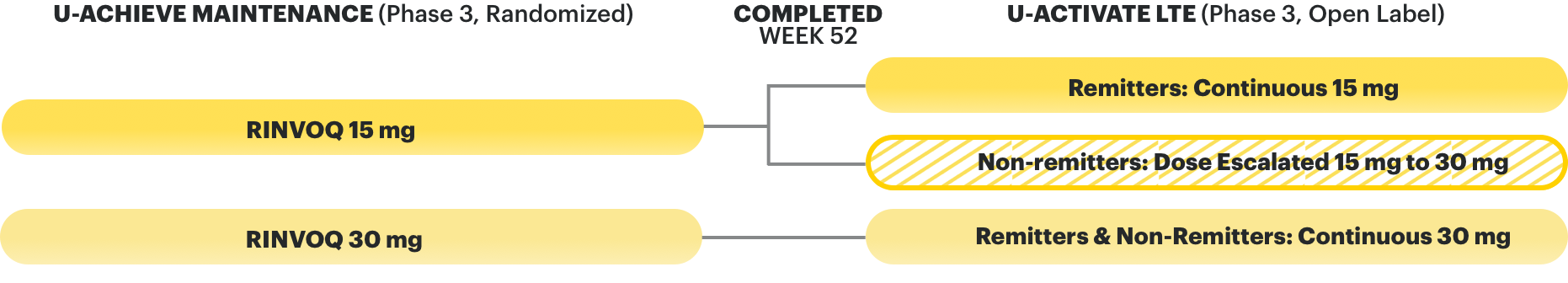
Dr. Oriana Damas: In addition, data is also available for RINVOQ’s open-label extension, the U-ACTIVATE study.
Narrator: In an open-label extension (OLE), there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out.
In an as-observed analysis, missing data were excluded from calculations from that visit, which may increase the percent of responders. All observed data were used regardless of premature discontinuation of study drug or initiation of concomitant medications. The same patient may not have a response at each time point.
Dr. Oriana Damas: All patients who completed the maintenance trial were eligible for the open-label extension trial. The RINVOQ 15-mg arm includes patients who were in remission at the completion of the U-ACHIEVE maintenance trial and taking continuous RINVOQ 15 mg. The 30-mg arm includes both patients who were in remission and patients who were not in remission from the maintenance trial regardless of remission status.
Narrator: The interim analysis includes 101 patients on continuous RINVOQ 15 mg and 175 patients on RINVOQ continuous 30 mg, following the U-ACHIEVE maintenance trial.
Dr. Oriana Damas: At Week 48, Week 100 overall, 82% of patients on continuous RINVOQ 15 mg and 78% of patients on continuous RINVOQ 30 mg observed endoscopic improvement.
The difference in remission status upon entrance to the U-ACTIVATE open-label long-term extension may explain the difference in efficacy between the 15- and 30-mg arms.
Dr. David Rubin: In addition to endoscopic improvement, the RINVOQ trials also measured endoscopic remission, defined by a subscore of 0 or a normal appearance of the colon. At Week 52, approximately a quarter of patients achieved endoscopic remission on RINVOQ compared with 6% on placebo. At Week 100 overall, 55% of patients on RINVOQ 15 mg and 51% on RINVOQ 30 mg achieved endoscopic remission.
Oriana, seeing these endoscopic data from the RINVOQ clinical trials, in your personal experience, do you find results are similar?
Dr. Oriana Damas: So, I prescribe the 30-mg dose of RINVOQ once daily for patients with refractory, severe, or extensive disease, and I have found that RINVOQ is an efficacious medicine that achieves endoscopic outcomes. When my RINVOQ patients are experiencing sustained symptom relief, we’re then able to do an endoscopy and confirm that they’re showing minimal signs of disease activity.
From its induction trials, through its maintenance trial, we see that RINVOQ delivered significant endoscopic improvement compared to placebo.
Dr. David Rubin: The RINVOQ trials also assessed histology, using the endpoint of histo-endoscopic mucosal improvement. Patients who achieved this stringent endpoint achieved both endoscopic improvement with a Mayo subscore of 0 or 1 without friability and histologic improvement with a Geboes score less than or equal to 3.1, indicating neutrophil infiltration in <5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue.
Now, we should note that the relationship between histo-endoscopic mucosal improvement to disease progression and long-term outcomes was not evaluated. That said, at Week 8, results from the U-ACHIEVE and U-ACCOMPLISH induction studies showed a powerful treatment difference, with one in three RINVOQ patients demonstrating visible improvement in the colon lining and decreased histologic inflammatory activity, as measured by the Geboes Index score.
At Week 52, 35% of patients on RINVOQ 15 mg and 50% of patients on RINVOQ 30 mg achieved histo-endoscopic mucosal improvement, with 12% on placebo achieving the same endpoint. Endoscopic results are based on a full colonoscopy or flexible sigmoidoscopy, depending on the extent of disease at study entry. And histology results are based on a set of two biopsies.
Dr. Oriana Damas: Now, that data really stands out to me. I mean, half of the 30-mg cohort was able to achieve both endoscopic and histologic improvement? That signals to me that RINVOQ is an efficacious medicine that has demonstrated the ability to not only deliver clinical remission, but meets important endpoints and adjunctive healing measures, playing a key role in our pursuit of controlled disease.
I have personally witnessed endoscopic and histologic improvement with RINVOQ.
Dr. David Rubin: Overall, my impression of RINVOQ remains that it is a proven treatment option for our moderate to severe UC patients and aligns with the STRIDE-II recommendations. I have patients who have been on RINVOQ for over 2 years, and these data also reflect what I am seeing in my practice.
Thank you so much, Oriana, for taking the time today to discuss the objective outcomes and the concept of healing for our patients with ulcerative colitis. I know we both look forward to seeing treatment options for UC continue to expand.
Dr. Oriana Damas: [Closing remarks and acknowledgment]
It was an absolute pleasure, and I hope that we hear more and learn more from your future guests.
Dr. David Rubin: If you’ve enjoyed this episode of Gut Reactions and found it educational, you can share it via the share button, email, LinkedIn, or on any of your preferred platforms. Until next time, this is Gut Reactions.
Narrator
IMPORTANT SAFETY INFORMATION
SERIOUS INFECTIONS
Patients treated with RINVOQ are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids. If a serious infection develops, interrupt RINVOQ until the infection is controlled.
Reported infections include:
- Active tuberculosis (TB), which may present with pulmonary or extrapulmonary disease. Test patients for latent TB before RINVOQ use and during therapy. Consider treatment for latent TB infection prior to RINVOQ use.
- Invasive fungal infections, including cryptococcosis and pneumocystosis.
- Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
Carefully consider the risks and benefits of treatment with RINVOQ prior to initiating therapy in patients with chronic or recurrent infection. Monitor patients closely for the development of signs and symptoms of infection during and after treatment with RINVOQ, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy.
MORTALITY
In a large, randomized, postmarketing safety study comparing another Janus kinase (JAK) inhibitor with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients greater than or equal to 50 years old with at least one cardiovascular (CV) risk factor, a higher rate of all-cause mortality, including sudden CV death, was observed with the JAK inhibitor. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ.
MALIGNANCIES
Lymphoma and other malignancies have been observed in patients treated with RINVOQ.
In a large, randomized, postmarketing safety study comparing another JAK inhibitor with TNF blockers in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]), lymphomas, and lung cancer (in current or past smokers) was observed with the JAK inhibitor. Patients who are current or past smokers are at additional increased risk.
With RINVOQ, consider the benefits and risks for the individual patient prior to initiating or continuing therapy, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers. NMSCs have been reported in patients treated with RINVOQ. Periodic skin examination is recommended for patients who are at increased risk for skin cancer. Advise patients to limit sunlight exposure by wearing protective clothing and using sunscreen.
MAJOR ADVERSE CARDIOVASCULAR EVENTS
In a large, randomized, postmarketing study comparing another JAK inhibitor with TNF blockers in RA patients greater than or equal to 50 years old with at least one CV risk factor, a higher rate of major adverse cardiovascular events (defined as cardiovascular death, myocardial infarction, and stroke) was observed with the JAK inhibitor. Patients who are current or past smokers are at additional increased risk. Discontinue RINVOQ in patients that have experienced a myocardial infarction or stroke.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ, particularly in patients who are current or past smokers and patients with other CV risk factors. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
THROMBOSIS
Thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated with JAK inhibitors used to treat inflammatory conditions. Many of these adverse events were serious and some resulted in death.
In a large, randomized, postmarketing study comparing another JAK inhibitor to TNF blockers in RA patients greater than or equal to 50 years old with at least one CV risk factor, a higher rate of thrombosis was observed with the JAK inhibitor. Avoid RINVOQ in patients at risk. Patients with symptoms of thrombosis should discontinue RINVOQ and be promptly evaluated.
HYPERSENSITIVITY
RINVOQ is contraindicated in patients with known hypersensitivity to upadacitinib or any of its excipients. Serious hypersensitivity reactions, such as anaphylaxis and angioedema, were reported in patients receiving RINVOQ in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue RINVOQ and institute appropriate therapy.
GASTROINTESTINAL PERFORATIONS
Gastrointestinal (GI) perforations have been reported in clinical trials with RINVOQ. Monitor RINVOQ-treated patients who may be at risk for gastrointestinal perforation (e.g., patients with a history of diverticulitis and patients taking NSAIDs or corticosteroids). Promptly evaluate patients presenting with new onset abdominal pain for early identification of GI perforation.
LABORATORY ABNORMALITIES
Neutropenia
Treatment with RINVOQ was associated with an increased incidence of neutropenia (absolute neutrophil count [ANC] less than 1000 cells per millimeter cubed). Treatment with RINVOQ is not recommended in patients with an ANC less than 1000 cells per millimeter cubed. Evaluate neutrophil counts at baseline and thereafter according to routine patient management.
Lymphopenia
Absolute lymphocyte counts (ALC) less than 500 cells per millimeter cubed were reported in RINVOQ-treated patients. Treatment with RINVOQ is not recommended in patients with an ALC less than 500 cells per millimeter cubed. Evaluate at baseline and thereafter according to routine patient management.
Anemia
Decreases in hemoglobin levels to less than 8 grams per deciliter were reported in RINVOQ-treated patients. Treatment should not be initiated or should be interrupted in patients with hemoglobin levels less than 8 grams per deciliter. Evaluate at baseline and thereafter according to routine patient management.
Lipids
Treatment with RINVOQ was associated with increases in lipid parameters, including total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol. Manage patients according to clinical guidelines for the management of hyperlipidemia. Evaluate patients 12 weeks after initiation of treatment and thereafter according to the clinical guidelines for hyperlipidemia.
Liver enzyme elevations
Treatment with RINVOQ was associated with increased incidence of liver enzyme elevation compared to placebo. Evaluate at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. If increases in aspartate aminotransferase or alanine aminotransferase are observed during routine patient management and drug-induced liver injury is suspected, RINVOQ should be interrupted until this diagnosis is excluded.
EMBRYO-FETAL TOXICITY
Based on findings in animal studies, RINVOQ may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with RINVOQ and for 4 weeks after the final dose. Verify pregnancy status of females of reproductive potential prior to starting treatment with RINVOQ.
VACCINATION
Avoid use of live vaccines during, or immediately prior to, RINVOQ therapy. Prior to initiating RINVOQ, patients should be brought up to date on all immunizations, including prophylactic varicella zoster or herpes zoster vaccinations, in agreement with current immunization guidelines.
MEDICATION RESIDUE IN STOOL
Reports of medication residue in stool or ostomy output have occurred in patients taking RINVOQ. Most reports described anatomic or functional GI conditions with shortened GI transit times. Instruct patients to contact their healthcare provider if medication residue is observed repeatedly. Monitor patients clinically and consider alternative treatment if there is an inadequate therapeutic response.
LACTATION
There are no data on the presence of RINVOQ in human milk, the effects on the breastfed infant, or the effects on milk production. Available data in animals have shown the excretion of RINVOQ in milk. Advise patients that breastfeeding is not recommended during treatment with RINVOQ and for 6 days after the last dose.
HEPATIC IMPAIRMENT
RINVOQ is not recommended for use in patients with severe hepatic impairment.
ADVERSE REACTIONS
The most common adverse reactions in RINVOQ clinical trials were upper respiratory tract infections, herpes zoster, herpes simplex, bronchitis, nausea, cough, pyrexia, acne, headache, increased blood creatine phosphokinase, hypersensitivity, folliculitis, abdominal pain, increased weight, influenza, fatigue, neutropenia, myalgia, influenza-like illness, elevated liver enzymes, rash, and anemia.
Inform patients that retinal detachment has been reported in clinical trials with RINVOQ. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision while receiving RINVOQ.
Dosage Forms and Strengths: RINVOQ is available in 15 mg, 30 mg, and 45 mg extended-release tablets.