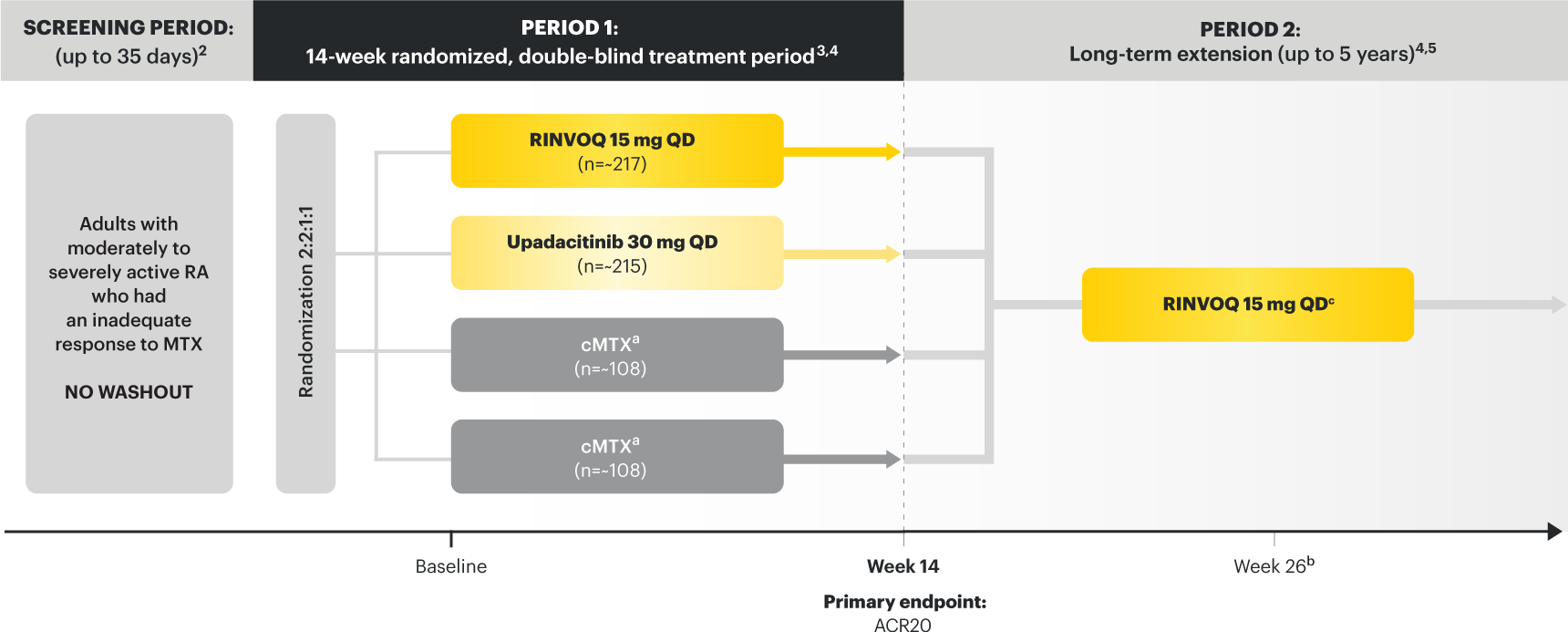
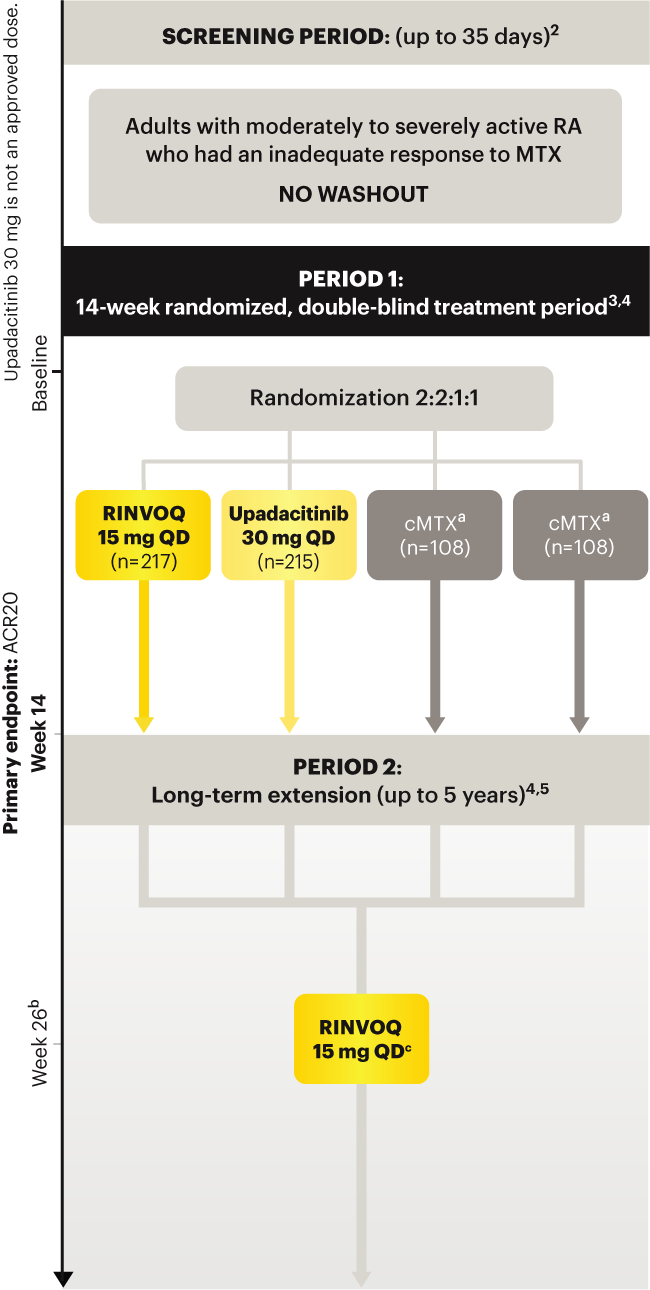
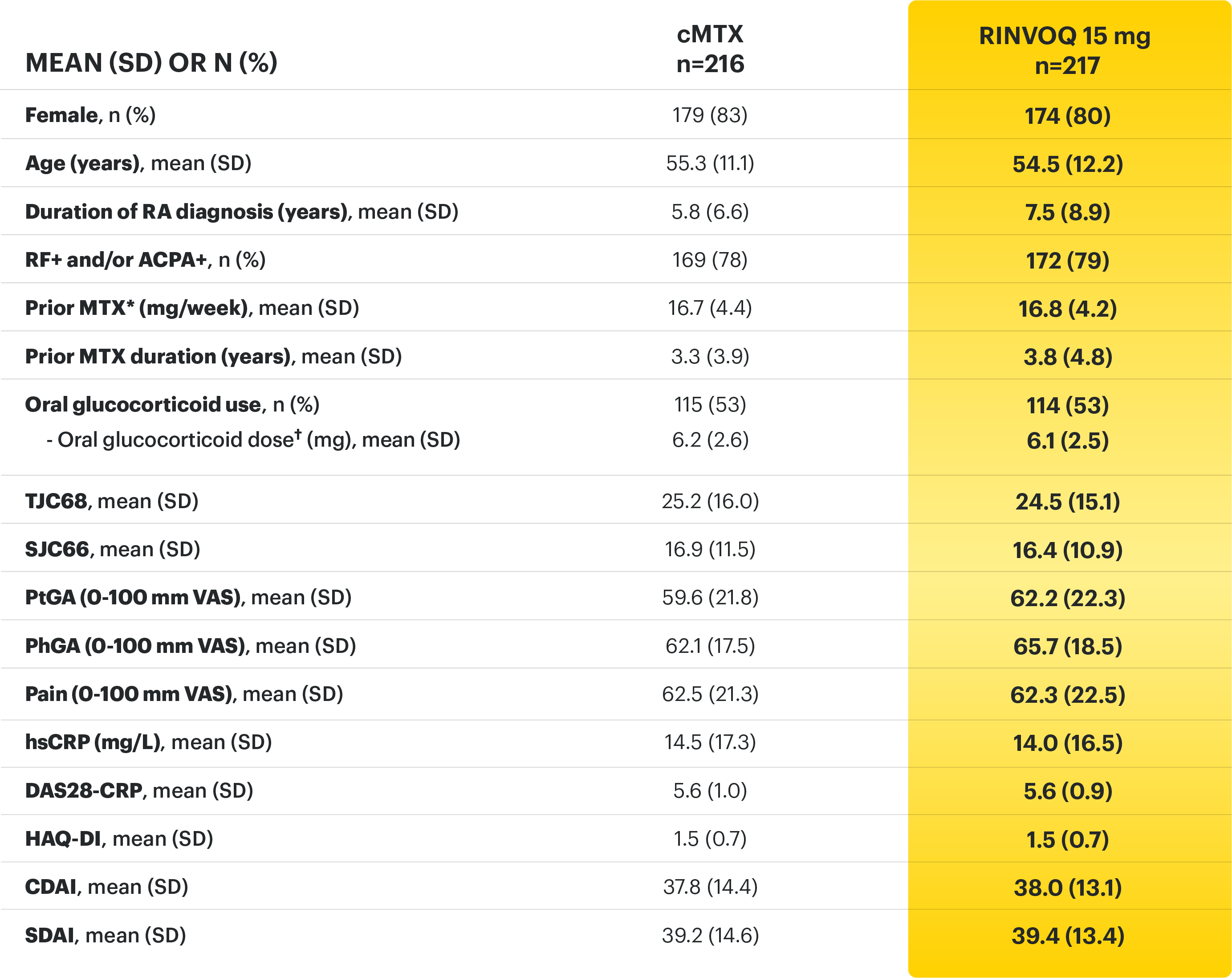
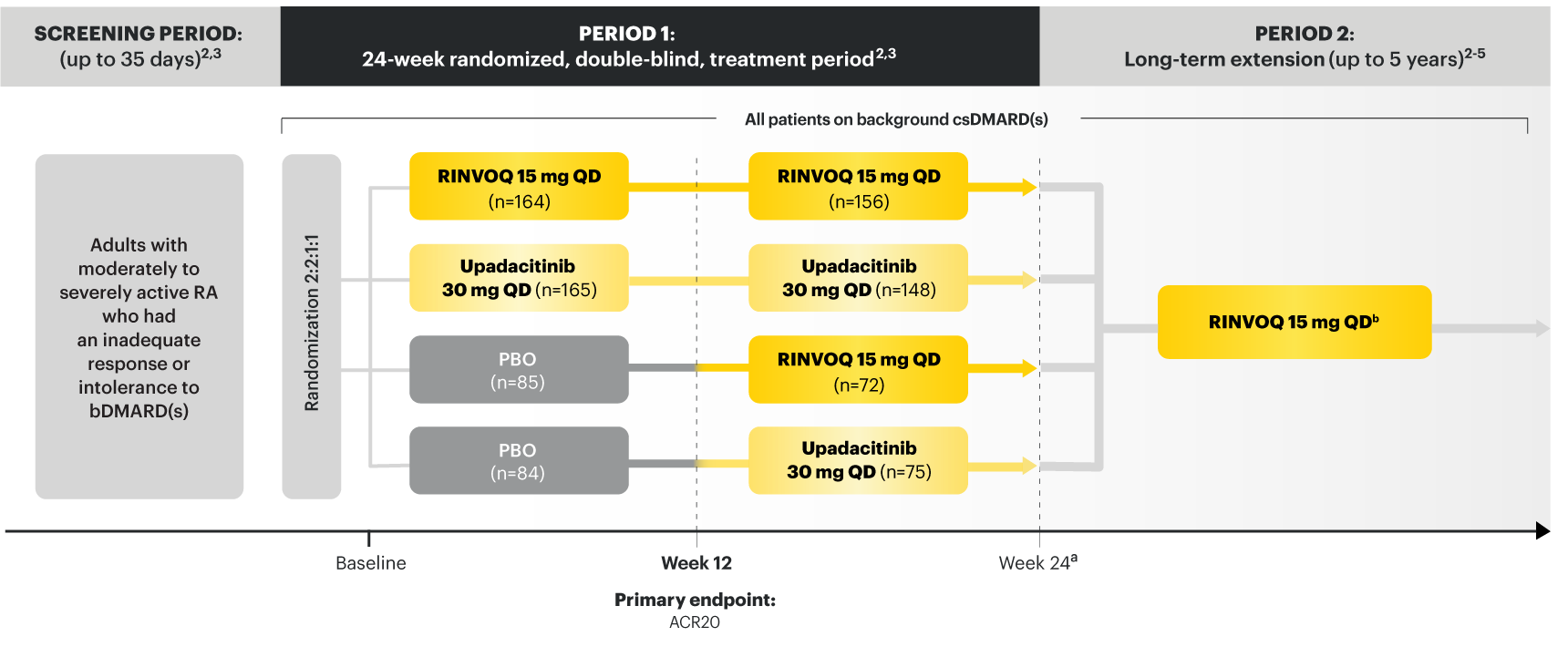
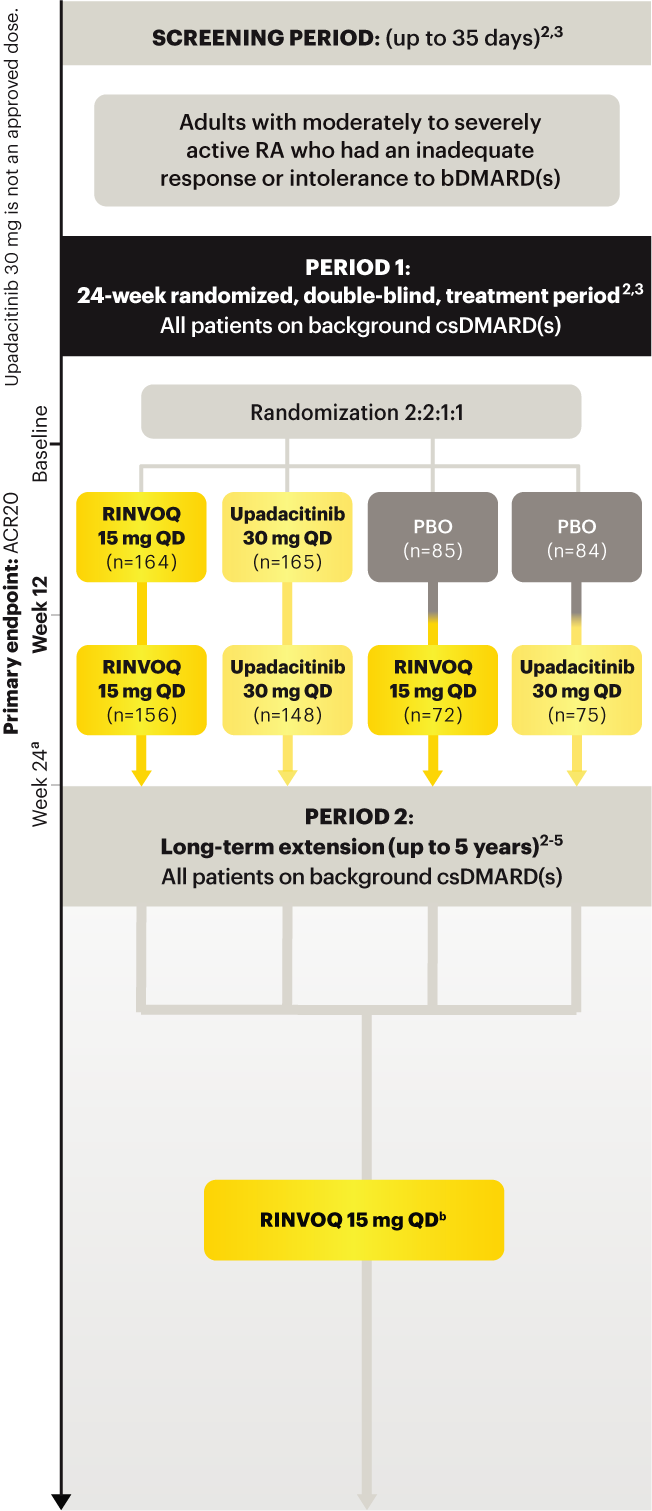
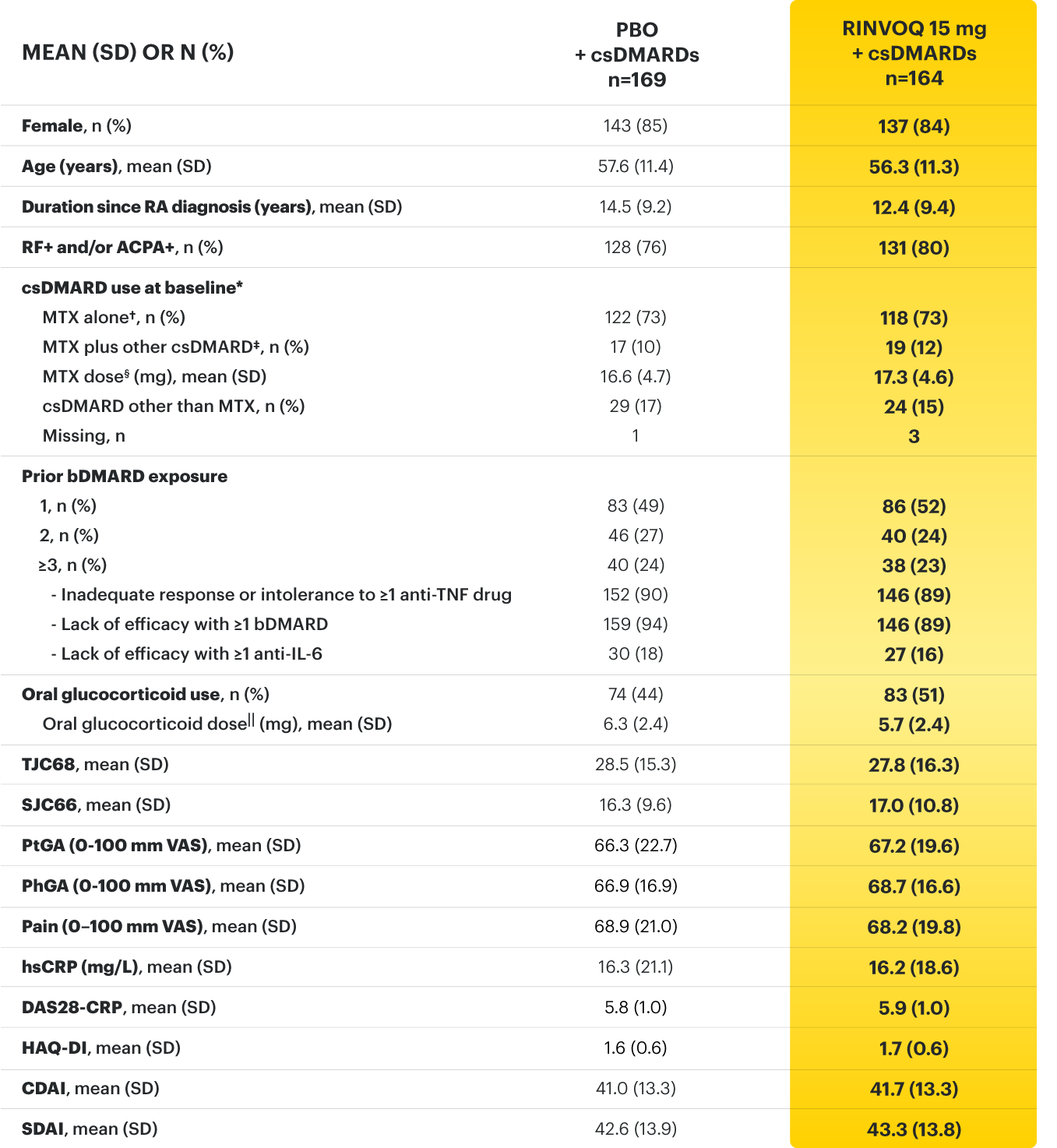
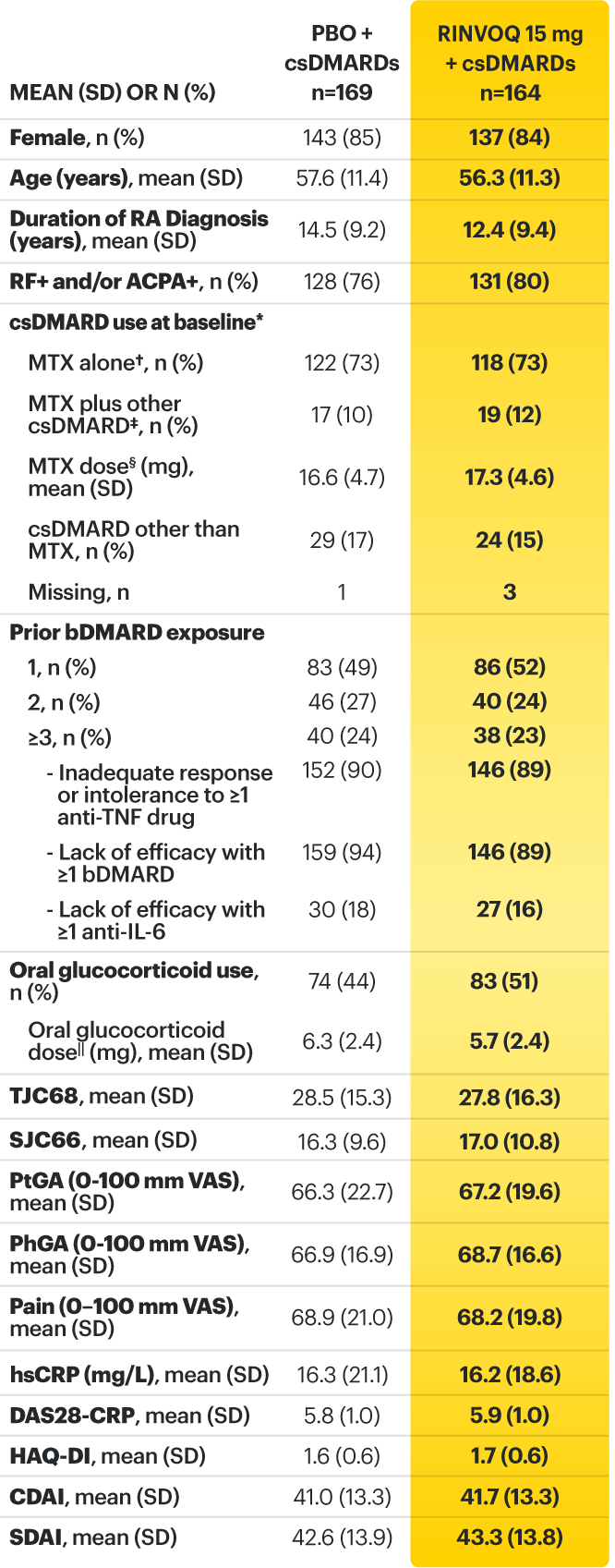
On January 14, 2022, the Prescribing Information and Medication Guide for RINVOQ (upadacitinib) was updated to include a new Indication, Recommended Dosage information and added a Contraindication related to Hypersensitivity and a new Warning and Precaution for Hypersensitivity Reactions.
The relevant sections of the Prescribing Information read as follows:
1 INDICATION AND USAGE
Section 1.3 Atopic Dermatitis
RINVOQ is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate to severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies are inadvisable.
Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
2 DOSAGE AND ADMINISTRATION
2.5 Recommended Dosage in Atopic Dermatitis
Pediatric Patients 12 Years of Age and Older Weighing at Least 40 kg and Adults Less Than 65 Years of Age
Initiate treatment with 15 mg once daily. If an adequate response is not achieved, consider increasing the dosage to 30 mg once daily. Discontinue RINVOQ if an adequate response is not achieved with the 30 mg dose. Use the lowest effective dose needed to maintain response.
Adults 65 Years of Age and Older
The recommended dosage is 15 mg once daily.
2.6 Recommended Dosage in Patients with Renal Impairment or Severe Hepatic Impairment
Renal Impairment
Atopic Dermatitis:
- For patients with severe renal impairment [creatinine clearance (CrCL) < 30 mL/min] the recommended dosage is 15 mg once daily.
- No dosage adjustment is needed for patients with mild or moderate renal impairment [(CrCL) > 30 mL/min)].
Hepatic Impairment
RINVOQ is not recommended for use in patients with severe hepatic impairment.
4 CONTRAINDICATIONS
RINVOQ is contraindicated in patients with known hypersensitivity to upadacitinib or any of its excipients.
5 WARNINGS AND PRECAUTIONS
Section 5.6 Hypersensitivity Reactions
Serious hypersensitivity reactions such as anaphylaxis and angioedema were reported in patients receiving RINVOQ in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue RINVOQ and institute appropriate therapy.
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise patients to discontinue RINVOQ and seek immediate medical attention if they develop any signs and symptoms of allergic reactions.
On December 2, 2021, the Prescribing Information and Medication Guide for RINVOQ® (upadacitinib) was updated as a result of discussions with the U.S. Food and Drug Administration (FDA). This follows a Drug Safety Communication (DSC) issued on September 1, 2021, by the FDA based upon its review of a large, randomized, post-marketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another Janus kinase (JAK) inhibitor to tumor necrosis factor (TNF) blockers.
The indication has been updated to the following:
1 INDICATION AND USAGE
Section 1.1 Rheumatoid Arthritis
RINVOQ (upadacitinib) is indicated for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to one or more TNF blockers.
Limitation of Use: Use of RINVOQ in combination with other JAK inhibitors, biologic DMARDs, or with potent immunosuppressants such as azathioprine and cyclosporine is not recommended.
The full Prescribing Information now includes new information about the risks of Mortality and Major Adverse Cardiovascular Events and updated information about the risks of Malignancies and Thrombosis within the Boxed Warning and Warnings and Precautions sections.
The Boxed Warning has been updated to the following:
The following sub-sections in the Warnings and Precautions have been updated to the following:
5 WARNINGS AND PRECAUTIONS
Section 5.2 Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ.
Section 5.3 Malignancy and Lymphoproliferative Disorders
Malignancies were observed in clinical studies of RINVOQ.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Non-Melanoma Skin Cancer
NMSCs have been reported in patients treated with RINVOQ. Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
Section 5.4 Major Adverse Cardiovascular Events
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue RINVOQ in patients that have experienced a myocardial infarction or stroke.
Section 5.5 Thrombosis
Thrombosis, including deep venous thrombosis (DVT), pulmonary embolism (PE), and arterial thrombosis, have occurred in patients treated for inflammatory conditions with JAK inhibitors, including RINVOQ. Many of these adverse events were serious and some resulted in death.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers.
If symptoms of thrombosis occur, patients should discontinue RINVOQ and be evaluated promptly and treated appropriately. Avoid RINVOQ in patients that may be at increased risk of thrombosis.
This information was added to the following section:
17 PATIENT COUNSELING INFORMATION
Major Adverse Cardiovascular Events
Inform patients that RINVOQ may increase their risk of major adverse cardiovascular events (MACE) including myocardial infarction, stroke, and cardiovascular death. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events.
The following information on important labeling revisions does not include all changes; please refer to the RINVOQ full Prescribing Information.
Indications and Important Safety Information for RINVOQ (upadacitinib)1