For active ankylosing spondylitis (AS) in adult TNFi-IR patients1
For active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation in adult TNFi-IR patients1
RAPID & DURABLE
DISEASE CONTROL
in AS1-3
RINVOQ achieved its primary endpoint of ASAS40 at Week 14 with responses observed at Week 4, up to 1 year1-3
RINVOQ achieved its primary endpoint of ASAS40 at Week 14 with responses observed at Week 4, up to 1 year1-3
AS=ankylosing spondylitis; ASAS=Assessment of SpondyloArthritis international Society; ASAS40=≥40% improvement and an absolute improvement from baseline of ≥2 units on a scale of 0 to 10 in at least 3 of the 4 domains, with no worsening in the fourth domain: total back pain, inflammation (mean score of BASDAI questions 5 and 6 on severity and duration of morning stiffness), physical function (BASFI), and Patient Global Assessment of disease activity; ASDAS=Ankylosing Spondylitis Disease Activity Score; BASDAI=Bath Ankylosing Spondylitis Disease Activity Index; BASDAI50=a 50% improvement of the initial Bath Ankylosing Spondylitis Disease Activity Index score; BASFI=Bath Ankylosing Spondylitis Functional Index; CRP=C-reactive protein; TNFi=tumor necrosis factor inhibitor
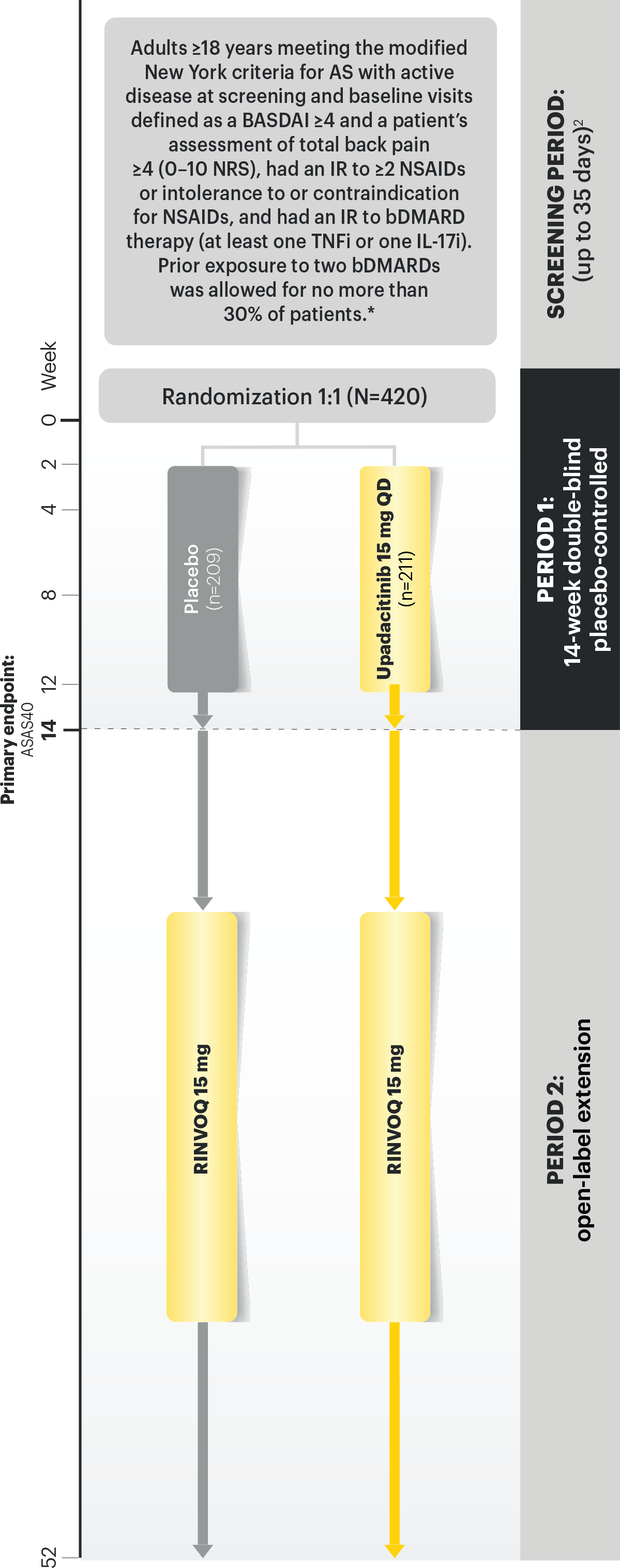
Clinical Trial Overview
*P<0.00012
SELECT-AXIS 2 Study 1: AS Design Intro:1,2
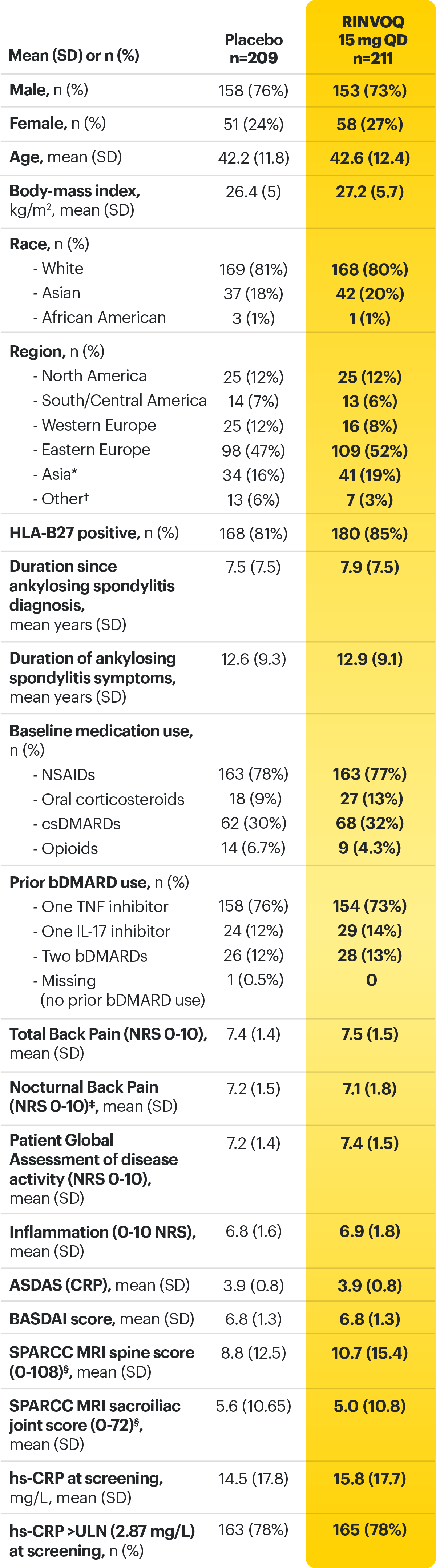
14-week, double-blind, parallel-group, placebo-controlled Phase 3 study of 420 patients with active AS who had an intolerance or inadequate response to at least two NSAIDs and 1 or 2 bDMARDs. Patients were randomized to receive RINVOQ 15 mg once daily or placebo. Patients could continue background NSAIDs. The primary endpoint was proportion of patients achieving ASAS40 response at Week 14 vs placebo.
AS=ankylosing spondylitis; ASAS40=≥40% improvement and an absolute improvement from baseline of ≥2 units on a scale of 0 to 10 in at least 3 of the 4 domains, with no worsening in the fourth domain: total back pain, inflammation (mean score of BASDAI questions 5 and 6 on severity and duration of morning stiffness), physical function (BASFI), and Patient Global Assessment of disease activity; BASDAI=Bath Ankylosing Spondylitis Disease Activity Index; BASFI=Bath Ankylosing Spondylitis Functional Index; bDMARD=biologic disease-modifying antirheumatic drug; DMARD=disease-modifying antirheumatic drug; NRI-MI=nonresponder imputation incorporating multiple imputation to handle missing data due to COVID-19; NSAID=nonsteroidal anti-inflammatory drug; QD=once per day; TNFi=tumor necrosis factor inhibitor
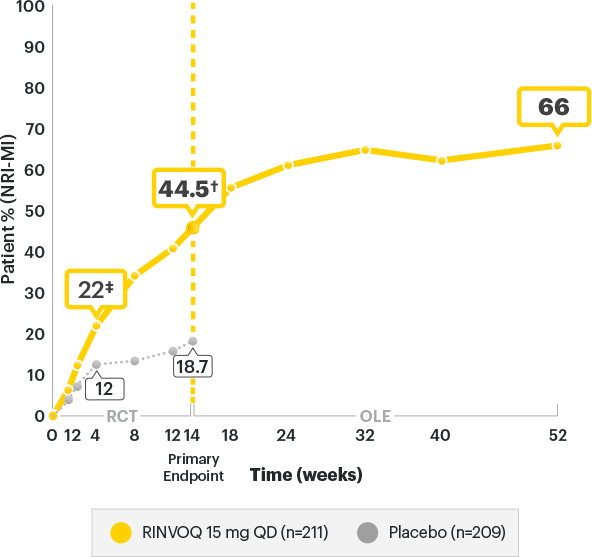
ASAS40 RATES1-3
RAPID Improvement in ASAS401,2
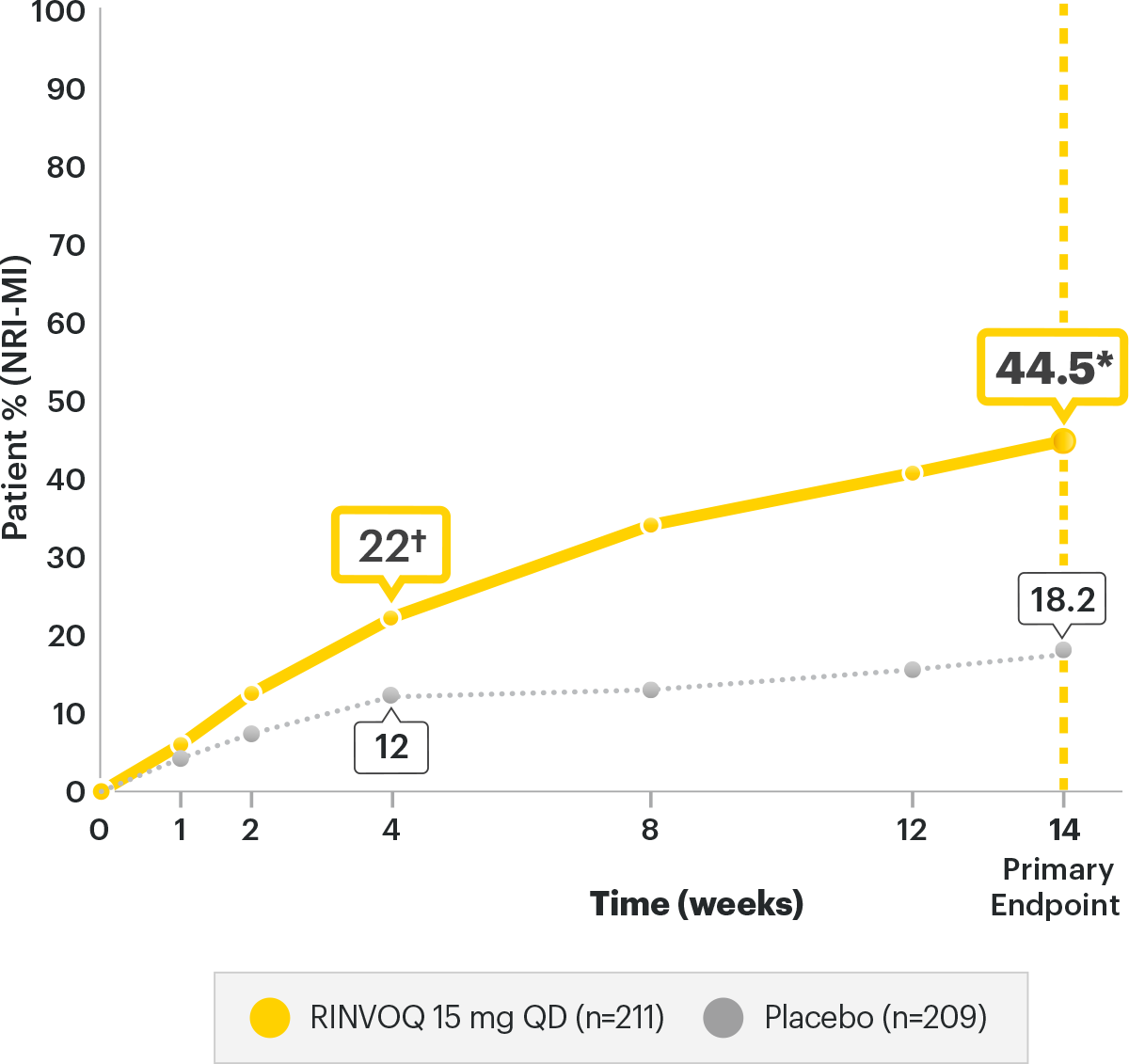
SELECT-AXIS 2 Study 1: AS ASAS40 Response
(NRI-MI)1,2
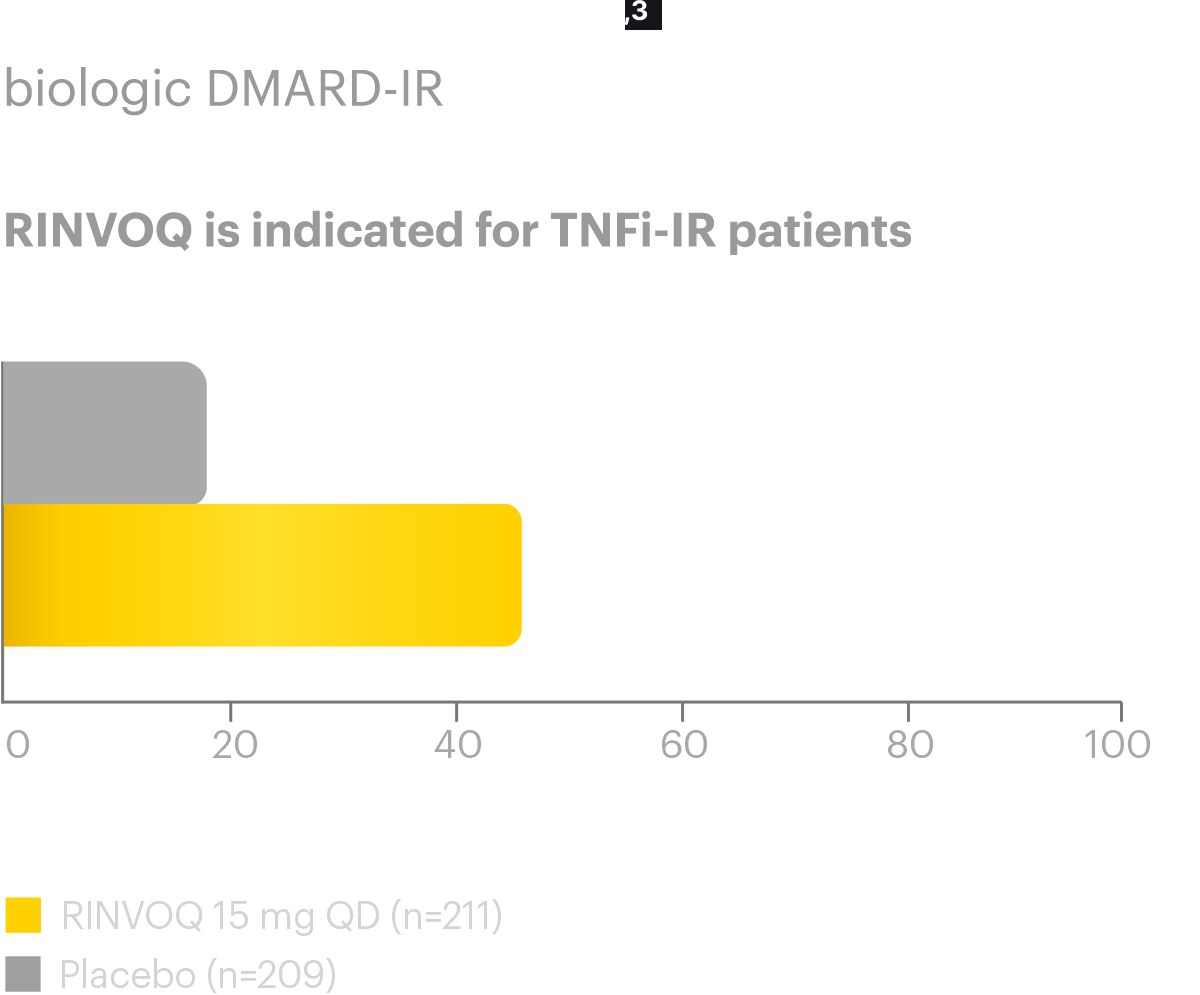
A study in biologic DMARD‑IR patients
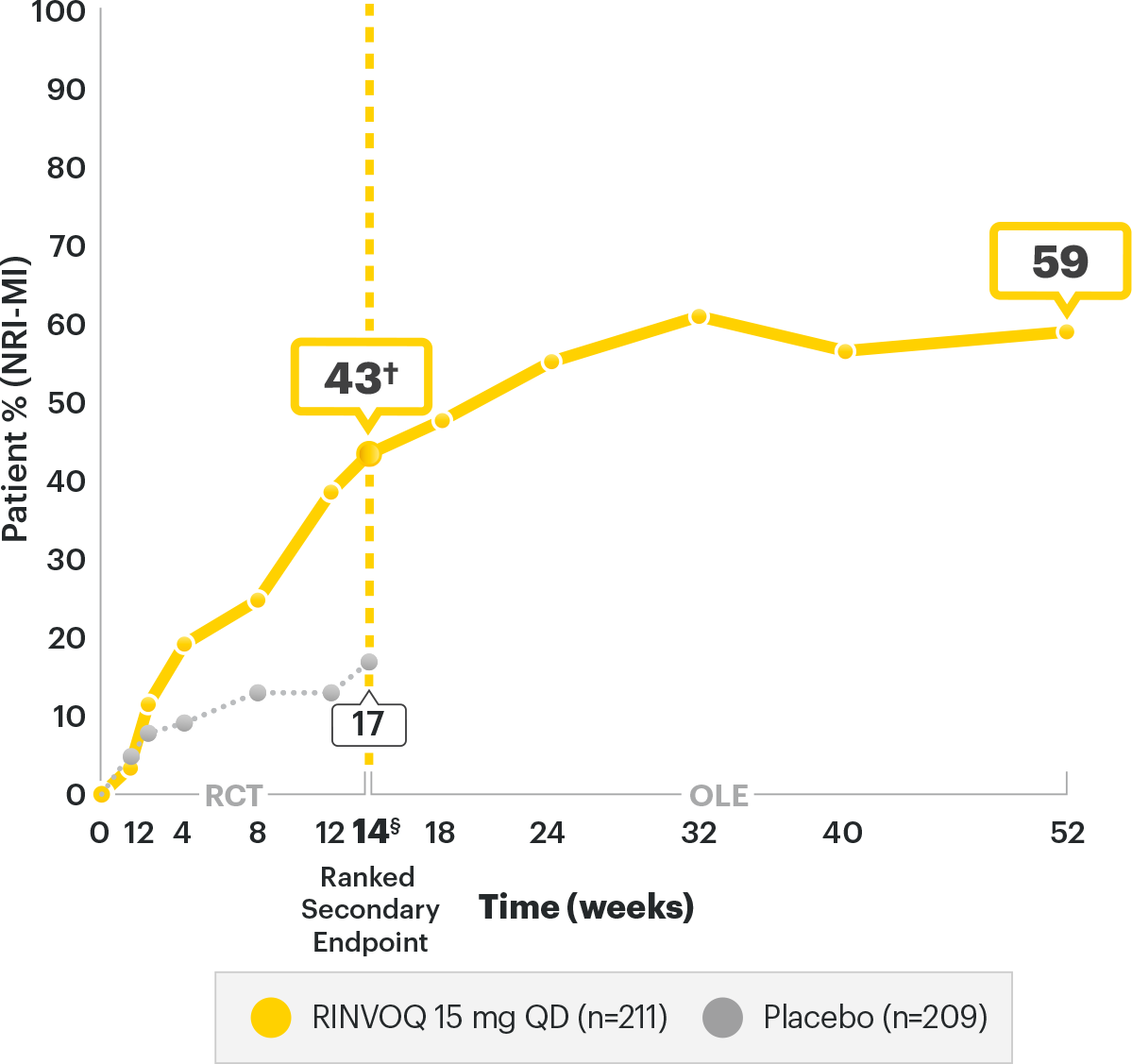
SELECT-AXIS 2 Study 1: AS ASAS40 Response (NRI-MI)1,2
A study in biologic DMARD‑IR patients
Nearly half (44.5%) of AS bDMARD-IR patients achieved ASAS40 primary endpoint at Week 14 (vs placebo
18.2%,P<0.0001)
Responses observed as early as 
RINVOQ is indicated for
TNFi-IR patients
*P<0.00012
†P≤0.05; P-value obtained through nominal statistical testing.2
ASAS40 reflects a ≥40% improvement in 3 of the 4 following domains with no worsening in the fourth domain:2
ASAS Domains
- Total back pain‡
- Inflammation (Morning Stiffness)§
- Physical function (BASFI)
- Patient Global Assessment of disease activity
‡Total back pain defined on an NRS (0-10) based on the following question: "What is the amount of back pain that you experienced at any time during the last week?"2
§Defined as mean of BASDAI questions 5 and 6.
Nearly half (44.5%) of AS bDMARD-IR patients achieved ASAS40 primary endpoint at Week 14 (vs placebo 18.2%,P<0.0001)
Responses observed as early as
ASAS40 reflects a ≥40% improvement in 3 of the 4 following domains with no worsening in the fourth domain:2
ASAS Domains
- Total back pain‡
- Inflammation (Morning Stiffness)§
- Physical function (BASFI)
- Patient Global Assessment of disease activity
‡Total back pain defined on an NRS (0-10) based on the following question: "What is the amount of back pain that you experienced at any time during the last week?"2
§Defined as mean of BASDAI questions 5 and 6.
Data LIMITATIONS:2 Data labeled as a primary endpoint at Week 14 were multiplicity-controlled. All other comparisons were not adjusted for multiplicity; therefore, statistical significance has not been established.
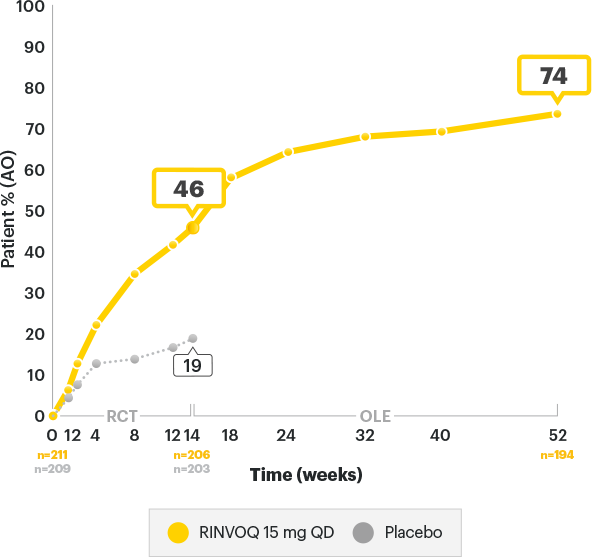
DURABLE ASAS40 Rates1-3
SELECT-AXIS 2 Study 1: AS ASAS40 Response up to Week 521-3
ALL DATA ARE OBSERVED CASES (AO)
A study in biologic DMARD‑IR patients
SELECT-AXIS 2 Study 1: AS ASAS40 Response up to Week 521-3
ALL DATA ARE OBSERVED CASES (AO)
A study in biologic DMARD‑IR patients
 of RINVOQ AS patients achieved ASAS40 at 1 year3
of RINVOQ AS patients achieved ASAS40 at 1 year3
RINVOQ is indicated for
TNFi-IR patients
ASAS40 reflects a ≥40% improvement in 3 of the 4 following domains with no worsening in the fourth domain:2
ASAS Domains
- Total back pain‡
- Inflammation (Morning Stiffness)§
- Physical function (BASFI)
- Patient Global Assessment of disease activity
‡Total back pain defined on an NRS (0-10) based on the following question: "What is the amount of back pain that you experienced at any time during the last week?"2
§Defined as mean of BASDAI questions 5 and 6.

of RINVOQ AS patients achieved ASAS40 at 1 year3
ASAS40 reflects a ≥40% improvement in 3 of the 4 following domains with no worsening in the fourth domain:2
ASAS Domains
- Total back pain‡
- Inflammation (Morning Stiffness)§
- Physical function (BASFI)
- Patient Global Assessment of disease activity
‡Total back pain defined on an NRS (0-10) based on the following question: "What is the amount of back pain that you experienced at any time during the last week?"2
§Defined as mean of BASDAI questions 5 and 6.
In an As Observed (AO) analysis, patients with missing data at a specific time are not included, which may enrich the population and increase the response rates.
OLE LIMITATIONS: There is potential for enrichment of OLE data; unblinding patients may cause bias related to overall treatment effect.
SELECT-AXIS 2 Study 1: AS ASAS40 Response up to Week 52 (NRI-MI)1-3
A study in biologic DMARD‑IR patients
SELECT-AXIS 2 Study 1: AS ASAS40 Response up to Week 52 (NRI-MI)1-3
A study in biologic DMARD‑IR patients
RINVOQ is indicated for
TNFi-IR patients
ASAS40 reflects a ≥40% improvement in 3 of the 4 following domains with no worsening in the fourth domain:2
ASAS Domains
- Total back pain§
- Inflammation (Morning Stiffness)‖
- Physical function (BASFI)
- Patient Global Assessment of disease activity
†P<0.00012,3
‡P<0.05;P-value obtained through nominal statistical testing2,3
§Total back pain defined on an NRS (0-10) based on the following question: "What is the amount of back pain that you experienced at any time during the last week?"2
‖Defined as mean of BASDAI questions 5 and 6.
ASAS40 reflects a ≥40% improvement in 3 of the 4 following domains with no worsening in the fourth domain:2
ASAS Domains
- Total back pain§
- Inflammation (Morning Stiffness)‖
- Physical function (BASFI)
- Patient Global Assessment of disease activity
†P<0.00012,3
‡P<0.05; P-value obtained through nominal statistical testing2,3
§Total back pain defined on an NRS (0-10) based on the following question: "What is the amount of back pain that you experienced at any time during the last week?"2
‖Defined as mean of BASDAI questions 5 and 6.
Data LIMITATIONS: Data labeled as a primary endpoint at Week 14 were multiplicity controlled. All other comparisons were not adjusted for multiplicity; therefore, statistical significance has not been established.
OLE LIMITATIONS: There is potential for enrichment of OLE data; unblinding patients may cause bias related to overall treatment effect.
ASDAS-LOW DISEASE ACTIVITYRATES2,3
ASDAS-LDA is a composite measure of disease activity in AS that assess common patient symptoms and an objective sign of inflammation.
ASDAS-CRP disease activity state components:4
A patient's ASDAS-CRP score is calculated by assessing the following components on a numerical rating scale (from 0 to 10):
- Back Pain
- Duration of Morning Stiffness
- Patient Global Assessment
- Peripheral Joint Pain/Swelling
- Inflammation (CRP)
ASDAS Disease Activity States5
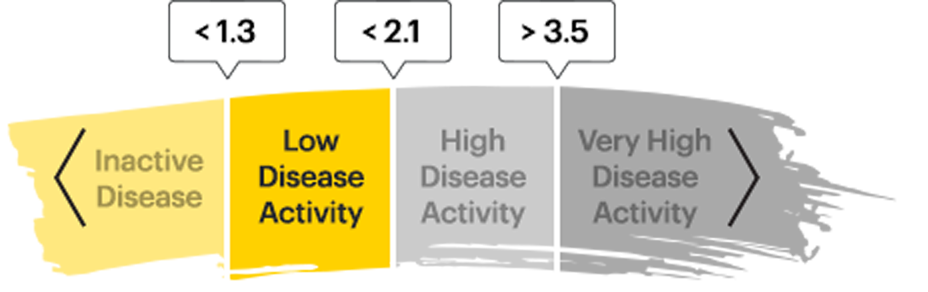
ASDAS low disease activity (LDA) is one of 4 disease activity states defined by the above cutoffs in the ASDAS score.

Achieving ASDAS-LDA may indicate disease control—making it a measure to strive for in patients with AS or nr-axSpA
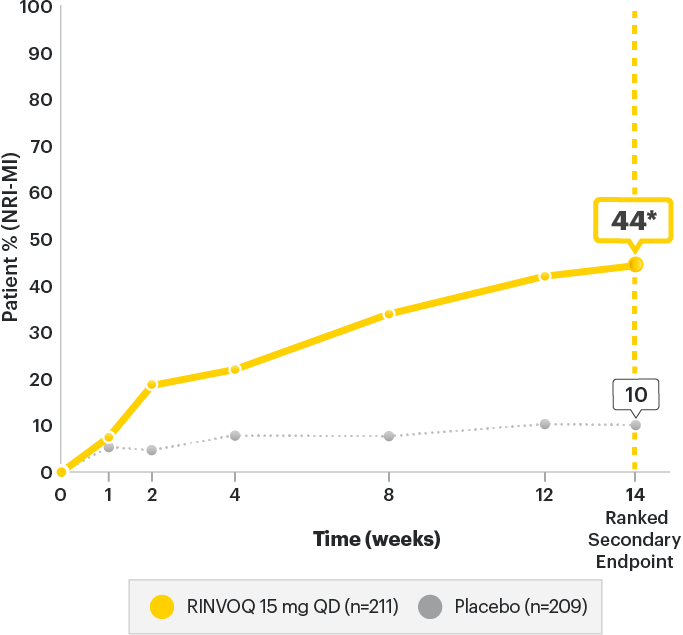
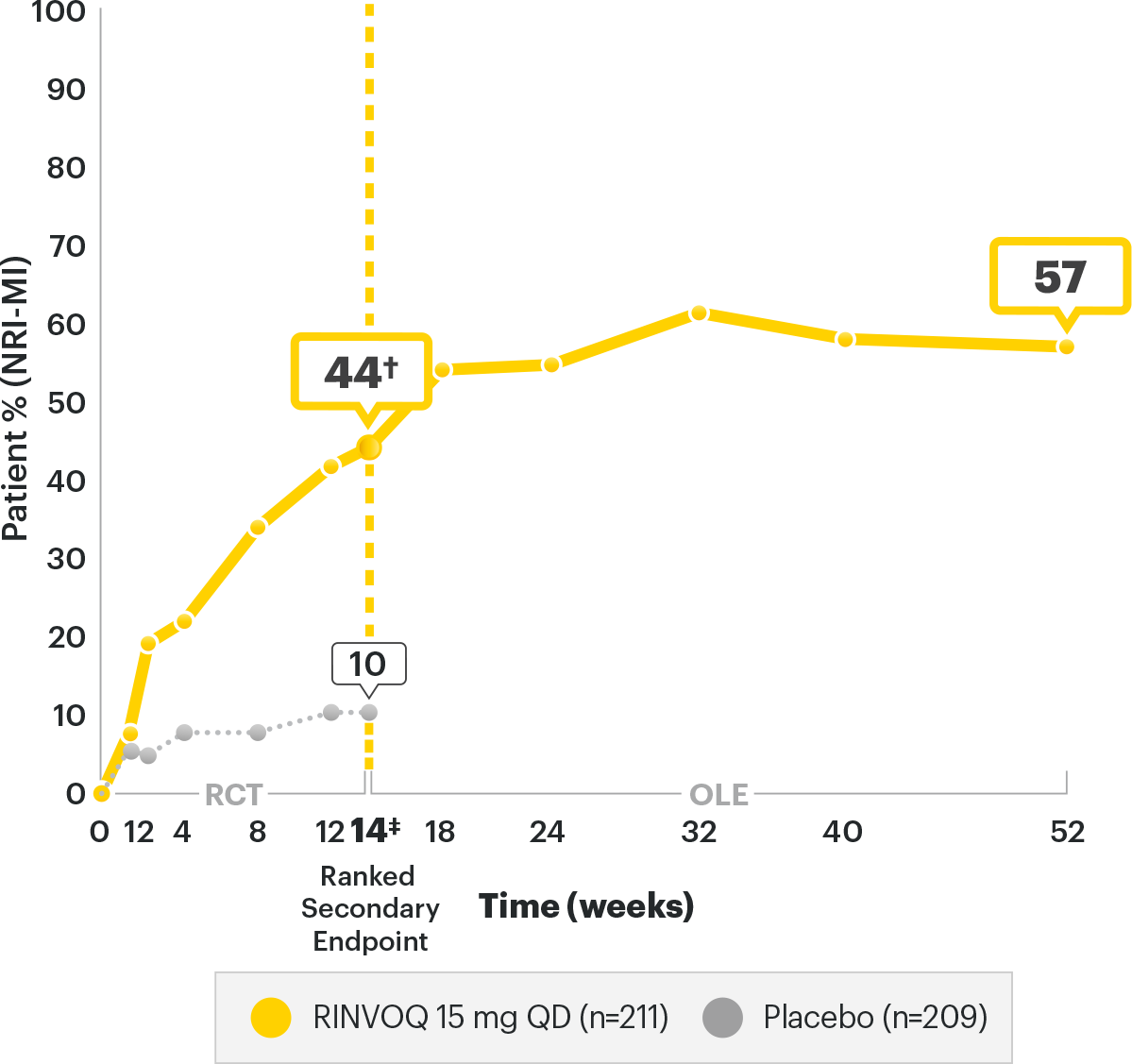
SELECT-AXIS 2 Study 1: AS ASDAS-CRP Low Disease Activity (LDA)
(NRI-MI)2
A study in biologic DMARD‑IR patients
SELECT-AXIS 2 Study 1: AS ASDAS-CRP Low Disease Activity (LDA) (NRI-MI)2
A study in biologic DMARD‑IR patients
Change from baseline (MMRM) ASDAS-CRP score:
RINVOQ is indicated for
TNFi-IR patients
*P<0.00012
| AS Patients | ||
|---|---|---|
| ASDAS-CRP Score | Placebo (n=209) |
RINVOQ (n=211) |
| Mean baseline | 3.9 | 3.9 |
| Change in baseline at Week 14 (MMRM) (Ranked Secondary Endpoint) |
-0.49 | -1.52 |
DATA LIMITATIONS:2 Data labeled as a ranked secondary endpoint at Week 14 were multiplicity-controlled. All other comparisons were not adjusted for multiplicity; therefore, statistical significance has not been established.
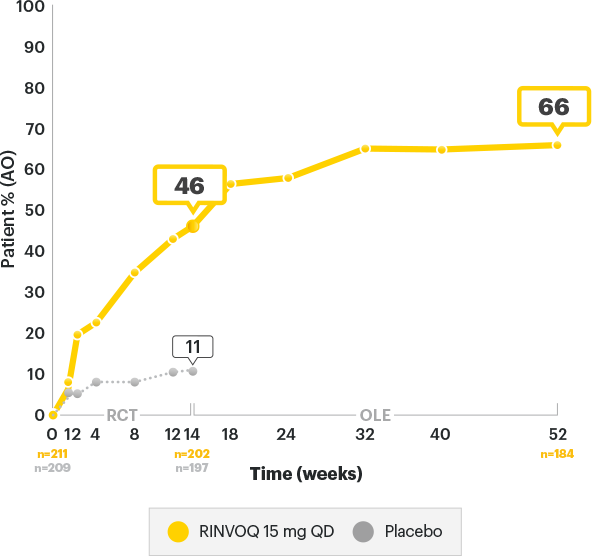
DURABLE ASDAS-CRP Low Disease Activity Rates3
SELECT-AXIS 2 Study 1: AS ASDAS-CRP Low Disease Activity (LDA) up to Week 523
ALL DATA ARE OBSERVED CASES (AO)
A study in biologic DMARD‑IR patients
SELECT-AXIS 2 Study 1: AS ASDAS-CRP Low Disease Activity (LDA) up to Week 523
ALL DATA ARE OBSERVED CASES (AO)
A study in biologic DMARD‑IR patients

of AS patients started in high (30.8%) or very high (68.7%) disease activity at baseline6
 of RINVOQ AS patients achieved Low Disease Activity at 1 year3
of RINVOQ AS patients achieved Low Disease Activity at 1 year3
RINVOQ is indicated for
TNFi-IR patients
In an As Observed (AO) analysis, patients with missing data at a specific time are not included, which may enrich the population and increase the response rates.
OLE LIMITATIONS: There is potential for enrichment of OLE data; unblinding patients may cause bias related to overall treatment effect.

of RINVOQ AS patients achieved Low Disease Activity at
1 year3
DURABLE ASDAS-CRP Low Disease Activity Rates3
SELECT-AXIS 2 Study 1: AS ASDAS-CRP Low Disease Activity (LDA) up to Week 52 (NRI-MI)3
A study in biologic DMARD‑IR patients
SELECT-AXIS 2 Study 1: AS ASDAS-CRP Low Disease Activity (LDA) up to Week 52 (NRI-MI)3
A study in biologic DMARD‑IR patients
RINVOQ is indicated for
TNFi-IR patients
†P<0.00012
DATA LIMITATIONS: Data labeled as a primary endpoint at Week 14 were multiplicity controlled. All other comparisons were not adjusted for multiplicity; therefore, statistical significance has not been established.
OLE LIMITATIONS: There is potential for enrichment of OLE data; unblinding patients may cause bias related to overall treatment effect.
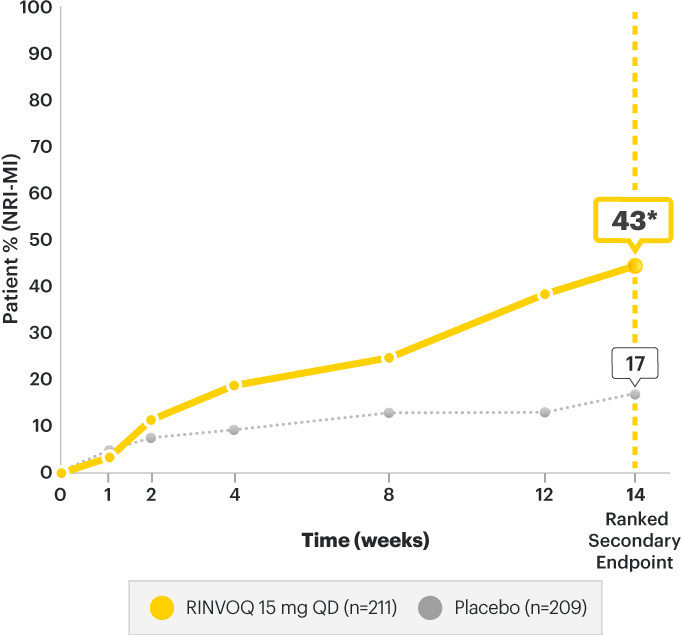
BASDAI50 RATES2,3
SELECT-AXIS 2 Study 1: AS BASDAI50 Response Rates (NRI-MI)2,3
A study in biologic DMARD‑IR patients
SELECT-AXIS 2 Study 1: AS BASDAI50 Response Rates (NRI-MI)2,3
A study in biologic DMARD‑IR patients
RINVOQ is indicated for
TNFi-IR patients
*P<0.00012
BASDAI50 reflects a ≥50% improvement in BASDAI from baseline7
BASDAI Components
- Fatigue
- Spinal pain
- Peripheral joint pain and swelling
- Enthesitis
- Severity of morning stiffness
- Duration of morning stiffness
*P<0.00012
BASDAI50 reflects a ≥50% improvement in BASDAI from baseline7
BASDAI Components
- Fatigue
- Spinal pain
- Peripheral joint pain and swelling
- Enthesitis
- Severity of morning stiffness
- Duration of morning stiffness
DATA LIMITATIONS:2 Data labeled as a ranked secondary endpoint at Week 14 were multiplicity-controlled. All other comparisons were not adjusted for multiplicity; therefore, statistical significance has not been established.
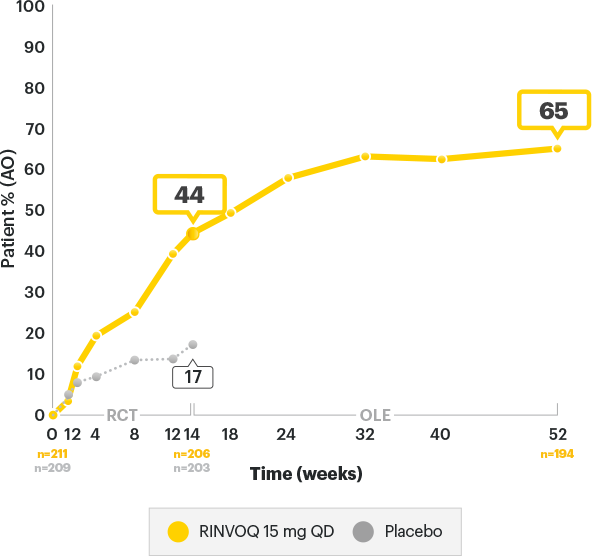
DURABLE BASDAI50 Rates2,3
SELECT-AXIS 2 Study 1: AS BASDAI50 Response Rates up to Week 523
ALL DATA ARE OBSERVED CASES (AO)
A study in biologic DMARD‑IR patients
SELECT-AXIS 2 Study 1: AS BASDAI50 Response Rates up to Week 523
ALL DATA ARE OBSERVED CASES (AO)
A study in biologic DMARD‑IR patients
 of RINVOQ AS patients achieved BASDAI50 at
of RINVOQ AS patients achieved BASDAI50 at
1 year3
RINVOQ is indicated for
TNFi-IR patients
BASDAI50 reflects a ≥50% improvement in BASDAI from baseline7
BASDAI Components
- Fatigue
- Spinal pain
- Peripheral joint pain and swelling
- Enthesitis
- Severity of morning stiffness
- Duration of morning stiffness
BASDAI50 reflects a ≥50% improvement in BASDAI from baseline7
BASDAI Components
- Fatigue
- Spinal pain
- Peripheral joint pain and swelling
- Enthesitis
- Severity of morning stiffness
- Duration of morning stiffness

of RINVOQ AS patients achieved BASDAI50 at 1 year3
In an As Observed (AO) analysis, patients with missing data at a specific time are not included, which may enrich the population and increase the response rates.
OLE LIMITATIONS: There is potential for enrichment of OLE data; unblinding patients may cause bias related to overall treatment effect.
DURABLE BASDAI50 Rates2,3
SELECT-AXIS 2 Study 1: AS BASDAI50 Response Rates up to Week 52
(NRI-MI)2,3
A study in biologic DMARD‑IR patients
SELECT-AXIS 2 Study 1: AS BASDAI50 Response Rates up to Week 52 (NRI-MI)2,3
A study in biologic DMARD‑IR patients
RINVOQ is indicated for
TNFi-IR patients
†P<0.00012
BASDAI50 reflects a ≥50% improvement in BASDAI from baseline7
BASDAI Components
- Fatigue
- Spinal pain
- Peripheral joint pain and swelling
- Enthesitis
- Severity of morning stiffness
- Duration of morning stiffness
†P<0.00014
BASDAI50 reflects a ≥50% improvement in BASDAI from baseline7
BASDAI Components
- Fatigue
- Spinal pain
- Peripheral joint pain and swelling
- Enthesitis
- Severity of morning stiffness
- Duration of morning stiffness
DATA LIMITATIONS: Data labeled as a primary endpoint at Week 14 were multiplicity controlled. All other comparisons were not adjusted for multiplicity; therefore, statistical significance has not been established.
OLE LIMITATIONS: There is potential for enrichment of OLE data; unblinding patients may cause bias related to overall treatment effect.
RINVOQ SAFETY DATA
Review the safety profile of RINVOQ,
including both short- and long-term analyses