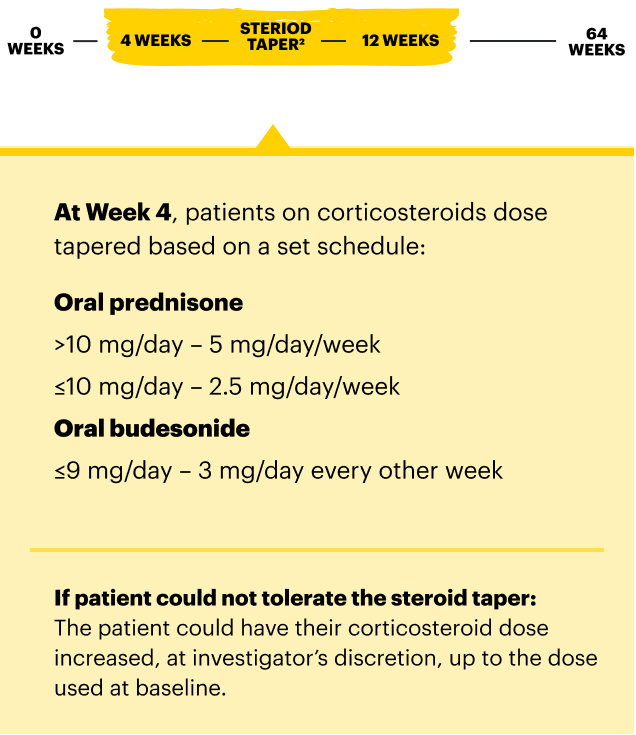
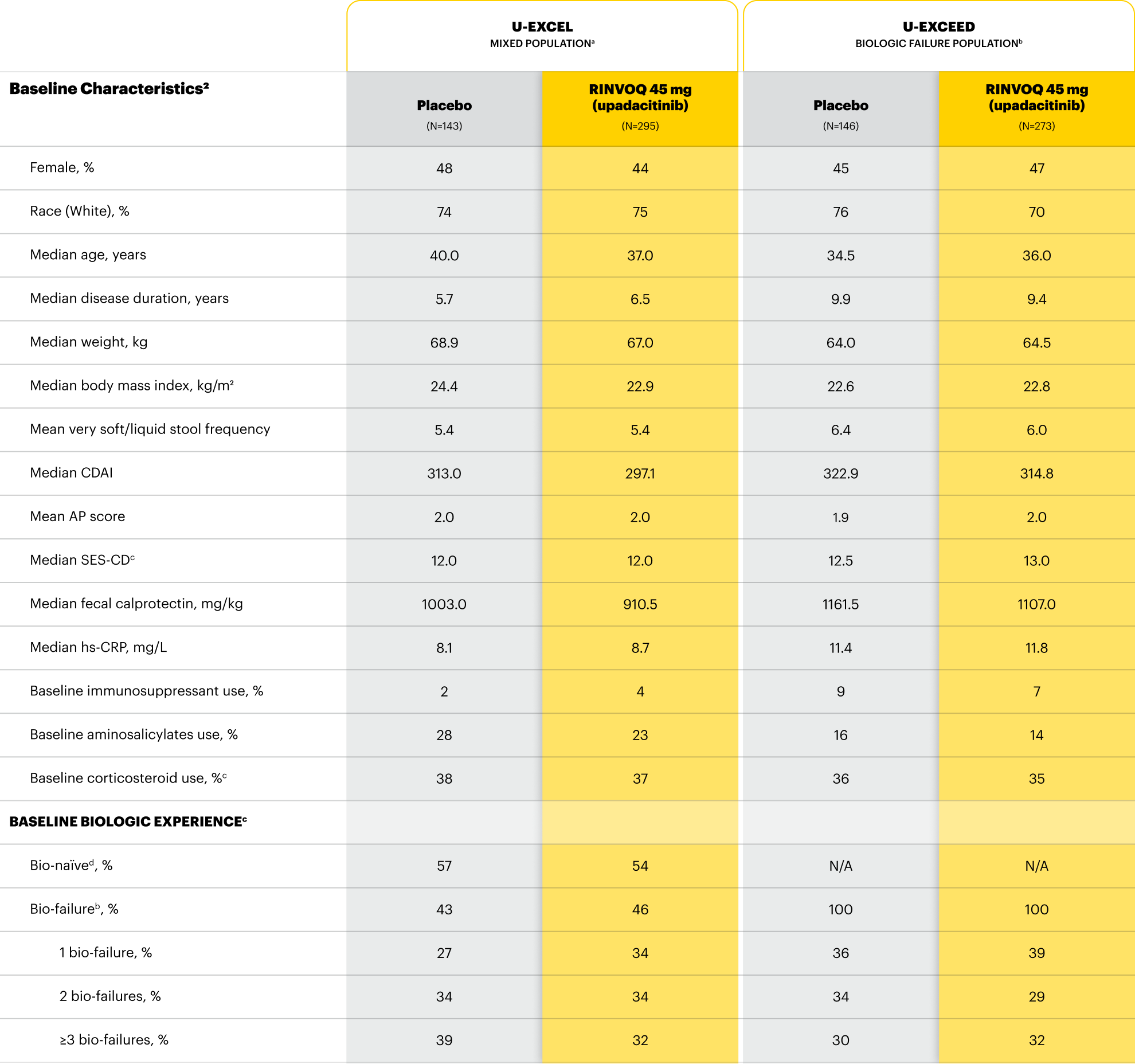
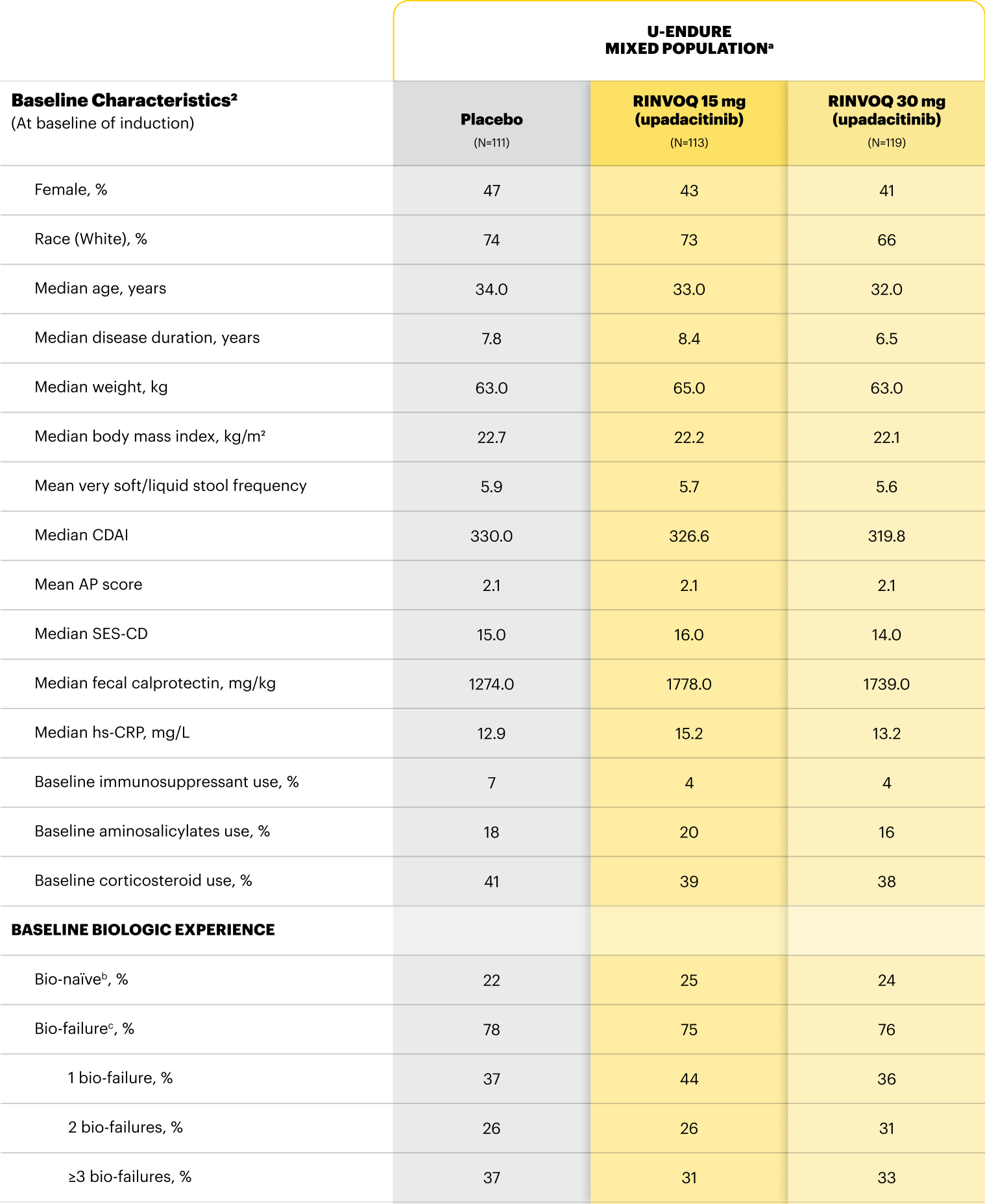
For moderate to severe Crohn's disease (CD) or moderate to severe ulcerative colitis (UC) in adult TNFi-IR patients.1
COMMITTED TO
EXCEPTIONAL
Get started with the RINVOQ Complete
Enrollment & Prescription Form
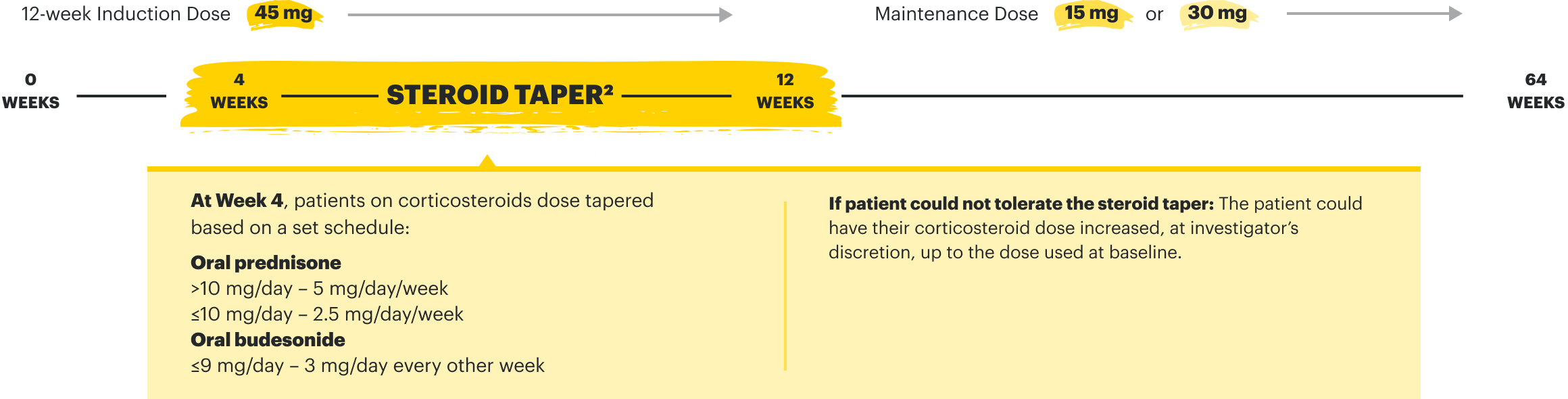
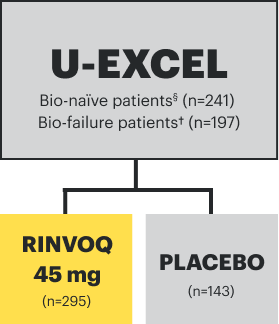
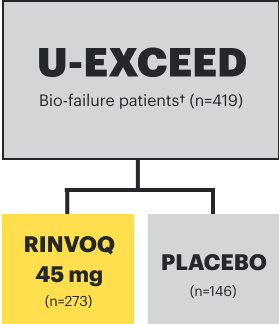
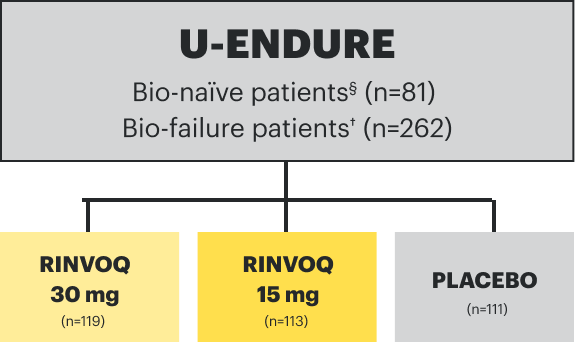
IR=intolerance or inadequate response;
TNFi=tumor necrosis factor inhibitor
Get started with the RINVOQ Complete Enrollment & Prescription Form
IR=intolerance or inadequate response;
TNFi=tumor necrosis factor inhibitor
RINVOQ HAS ACHIEVED EXCEPTIONAL ACCESS IN CROHN'S
IN CROHN'S
PREFERRED COMMERCIAL & MEDICARE PART D COVERAGE2*†
National commercial and Medicare Part D formulary coverage under the pharmacy
benefit as of September 2023
FIND COMMERCIAL INSURANCE PLANS FOR RINVOQ IN YOUR AREA.
Encourage your
patients to enroll in

ONE-TO-ONE SUPPORT
Nurse Ambassadors‡ and Access Specialists provide 1-to-1 support to help navigate insurance.
AFFORDABILITY
Eligible, commercially insured patients may pay as little as $0 per month on their prescription and can be reimbursed for the cost of related lab tests and monitoring.§
ACCESS
No charge for eligible patients experiencing initial insurance denial for up to 24 months.‖
STREAMLINED ENROLLMENT PROCESS
Get started with a single enrollment form.
Patients who are uninsured, or who are otherwise
unable to pay for their medication, may be eligible for:
*RINVOQ is on a preferred tier or otherwise has preferred status on the plan’s formulary.
†Coverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
‡Nurse Ambassadors are provided by AbbVie and do not provide medical advice or work under the direction of the prescribing health care professional (HCP). They are trained to direct patients to speak with their HCP about any treatment-related questions, including further referrals.
§Eligibility criteria: Available to patients with commercial insurance coverage for RINVOQ® (upadacitinib) who meet eligibility criteria. This co-pay assistance program is not available to patients receiving prescription reimbursement under any federal, state, or government-funded insurance programs (for example, Medicare [including Part D], Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs) or where prohibited by law. Offer subject to change or termination without notice. Restrictions, including monthly maximums, may apply. This is not health insurance. For full Terms and Conditions, visit RINVOQsavingscard.com or call 1-800-2RINVOQ (1-800-274-6867) for additional information. To learn about AbbVie’s privacy practices and your privacy choices, visit https://privacy.abbvie
‖Eligibility criteria: Available to patients aged 63 or younger with commercial insurance coverage. Patients must have a valid prescription for RINVOQ® (upadacitinib) for an FDA approved indication and a denial of insurance coverage based on a prior authorization request on file along with a confirmation of appeal. Continued eligibility for the program requires the submission of an appeal of the coverage denial every 180 days. Program provides for RINVOQ® (upadacitinib) at no charge to patients for up to two years or until they receive insurance coverage approval, whichever occurs earlier, and is not contingent on purchase requirements of any kind. Program is not available to patients whose medications are reimbursed in whole or in part by Medicare, Medicaid, TRICARE, or any other federal or state program. Offer subject to change or discontinuance without notice. This is not health insurance and program does not guarantee insurance coverage. No claims for payment may be submitted to any third party for product dispensed by program. Limitations may apply.
SUPPORTING
PATIENTS TOGETHER
AbbVie offers the COMPLETE Patient Support Programs with Specialists who provide 1-to-1
support to help patients throughout their prescribed treatment plan.
ACCESS
Dedicated specialists to assist with timely and affordable access to treatment
START
1-to-1 support to help ensure patients have the resources they need when they need them
STAY
Continuing support to help avoid potential treatment disruptions
HOW TO ENROLL PATIENTS IN RINVOQ COMPLETE
Help patients get the support they need to start and stay on track with their prescribed treatment.
GET STARTED WITH THE RINVOQ COMPLETE ENROLLMENT & PRESCRIPTION FORM
Help patients get the support they need to start and stay on track with their prescribed treatment.
FILL OUT THE FORM
WITH YOUR PATIENT
This form enrolls your patient and can be used to initiate a prescription with your patient’s preferred specialty pharmacy.
FILL OUT THE PATIENT SUPPORT
PRESCRIPTION IN SECTION 7
This may help your commercially insured patients get access to RINVOQ if they experience a delay or denial in their insurance coverage.*
FAX THE FORM
TO 1-678-727-0690
You will receive a call from an Access Specialist to discuss next steps. If using the Pharmacy Prescription in Section 6, fax a copy to your patient’s specialty pharmacy as well.
INFORM YOUR PATIENT THAT
THEY HAVE BEEN ENROLLED
Let your patient know that they will be receiving a call from their Nurse Ambassador.†
*Program is not available to patients receiving prescription reimbursement under any federal, state, or government-funded insurance programs (for example, Medicare [including Part D], Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs) or where prohibited by law or by the patient’s health insurance provider. If at any time a patient begins receiving prescription drug coverage under any such federal, state, or government-funded healthcare program, patient will no longer be eligible to participate in program. Available to patients between the ages of 18-63 with commercial prescription insurance coverage who meet eligibility criteria. Eligibility: Patients must be diagnosed with moderate to severe Crohn's disease, have a valid prescription for RINVOQ® and participate in a commercial insurance plan that has denied or not yet made a formulary decision for RINVOQ. Once the patient’s insurance plan has made a formulary decision and established a process for reviewing coverage requests for RINVOQ, continued eligibility for the program requires the submission of a Prior Authorization prior to the next scheduled dose and appeal of the coverage denial within 180 days. Program provides RINVOQ at no charge to patients for up to 2 years or until they receive insurance coverage approval, whichever occurs earlier. Offer subject to change or discontinuance without notice. This is not health insurance and program does not guarantee insurance coverage.
†Nurse Ambassadors are provided by AbbVie and do not provide medical advice or work under the direction of the prescribing healthcare professional (HCP). They are trained to direct patients to speak with their HCP about any treatment-related questions, including further referrals.
RINVOQ COMPLETE APP
Track treatment
The RINVOQ Complete App is designed to help patients stay on track with their prescribed RINVOQ treatment by helping them:
- Access additional resources, including a savings card for those who are eligible
- Set up medication reminders
- Log symptoms
- Log medication lot number and medication expiration date
COMPLETEPRO.COM
Streamline the Rx process
CompletePro.com enables seamless enrollment in RINVOQ Complete and helps streamline the prescription process for your patients.
With CompletePro.com, you can:
- Request benefits verifications
- Complete and submit prior authorizations
- Send prescriptions to the patient’s specialty pharmacy of choice, with the option to include a savings card
- Receive alerts for annual reauthorizations and renewals
- Track and monitor where patients are in the prescription process
LEARN MORE ABOUT
STREAMLINING THE
PRESCRIPTION PROCESS
WITH COMPLETE PRO
Download RINVOQ Resources
to support your patients and
your practice
INTERESTED IN LEARNING MORE
ABOUT THE SAFETY DATA?
See RINVOQ’s safety data
across clinical trials