For moderate to severe rheumatoid arthritis (RA) in adult TNFi-IR patients1
RA patients met ACR20 at Week 12 or 14 (Primary Endpoints) and Disease Control through Remission (DAS28-CRP <2.6)* at Weeks 12 or 14 and observed up to 5 years.1,3-6
*Clinical remission does not mean drug-free remission or complete absence of disease activity
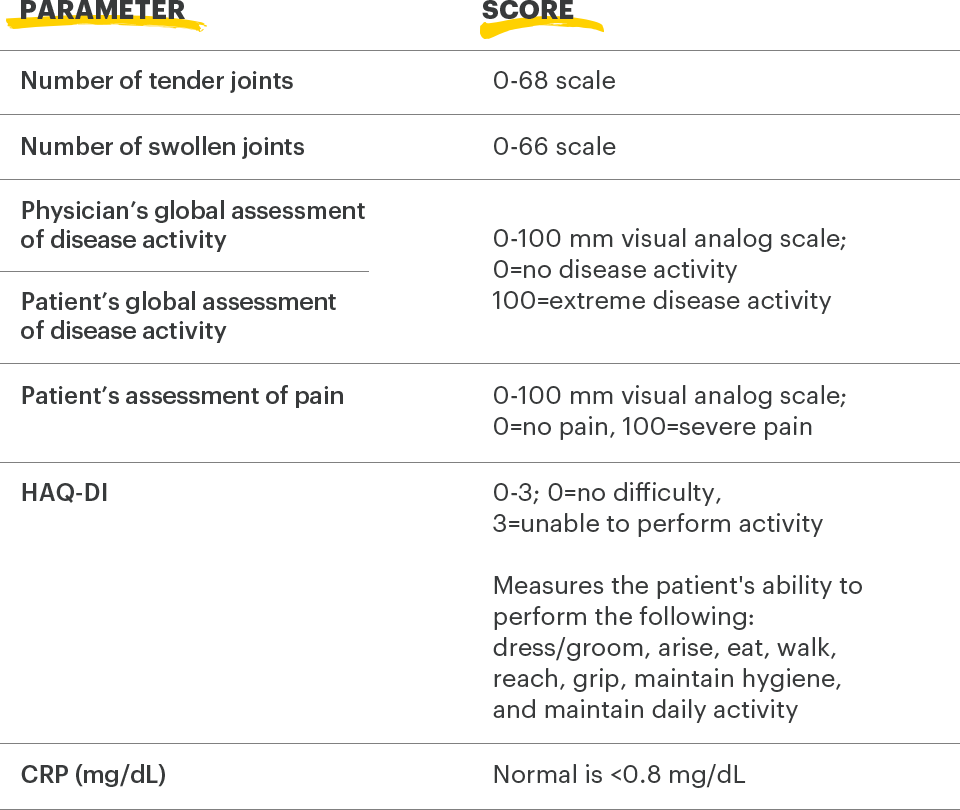
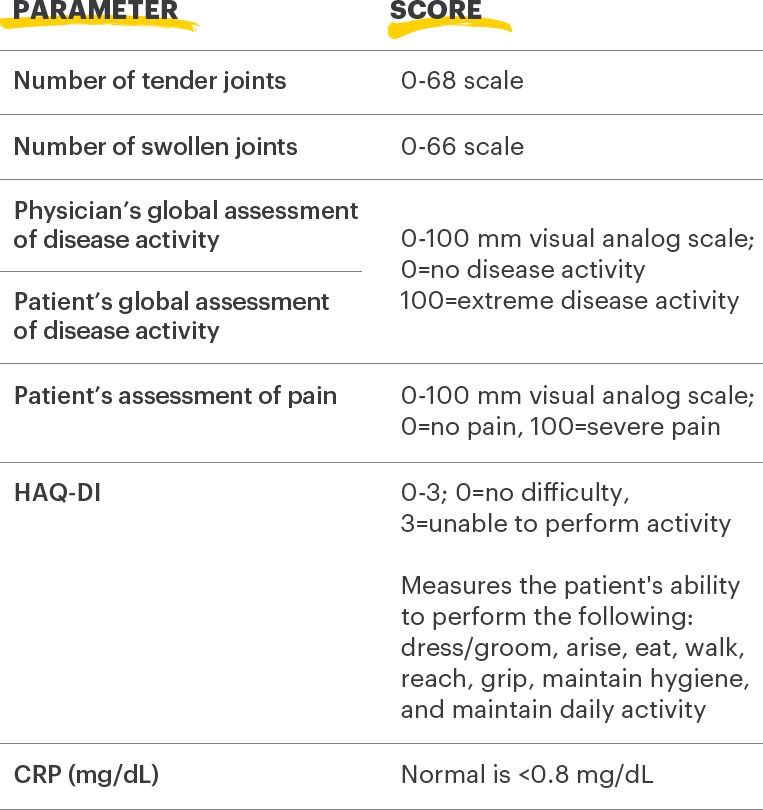
ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; CRP=C-reactive protein; DAS28-CRP=28 joint disease activity score using C-reactive protein

PAYERS COVER RINVOQ
PAYERS COVER RINVOQ
>95%
>95%
PREFERRED COMMERCIAL AND MEDICARE PART D COVERAGE2,*,†
PREFERRED COMMERCIAL AND MEDICARE PART D COVERAGE2,*,†
National Commercial and Medicare Part D formulary coverage under the pharmacy benefit as of January 2024.2
*RINVOQ is on a preferred tier or otherwise has preferred status on the plan’s formulary.
†Coverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
*RINVOQ is on a preferred tier or otherwise has preferred status on the plan’s formulary.
†Coverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.

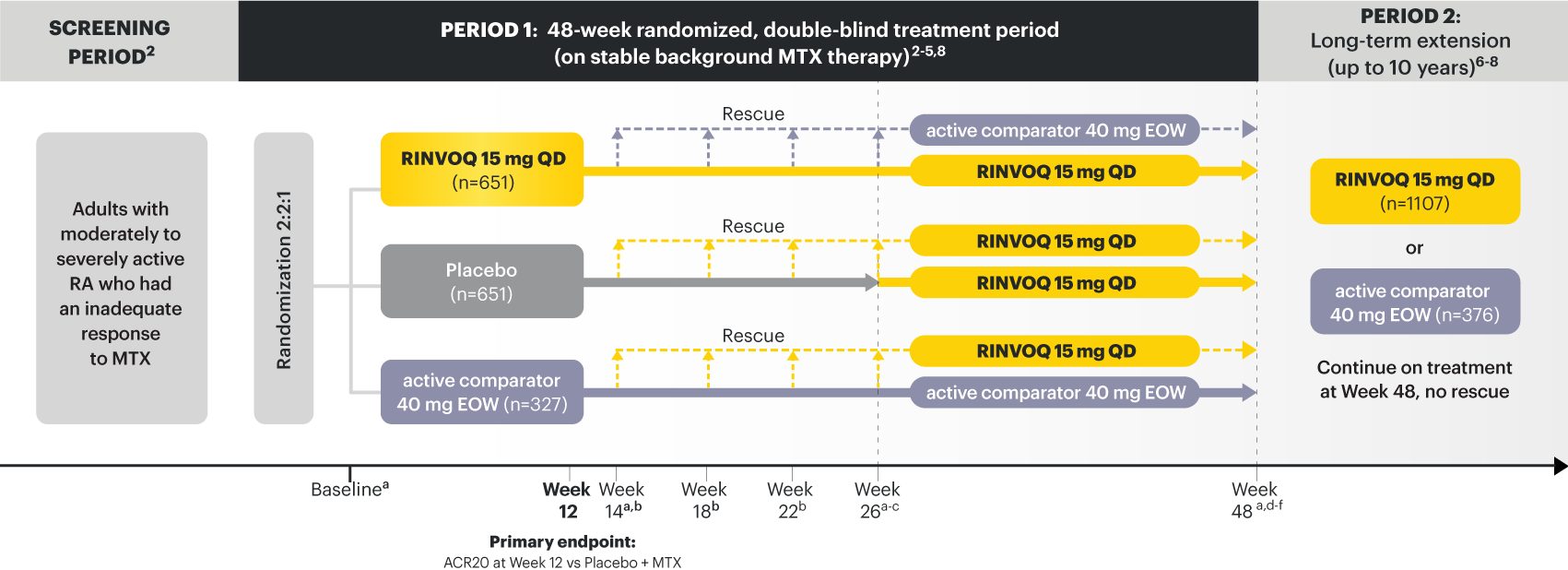
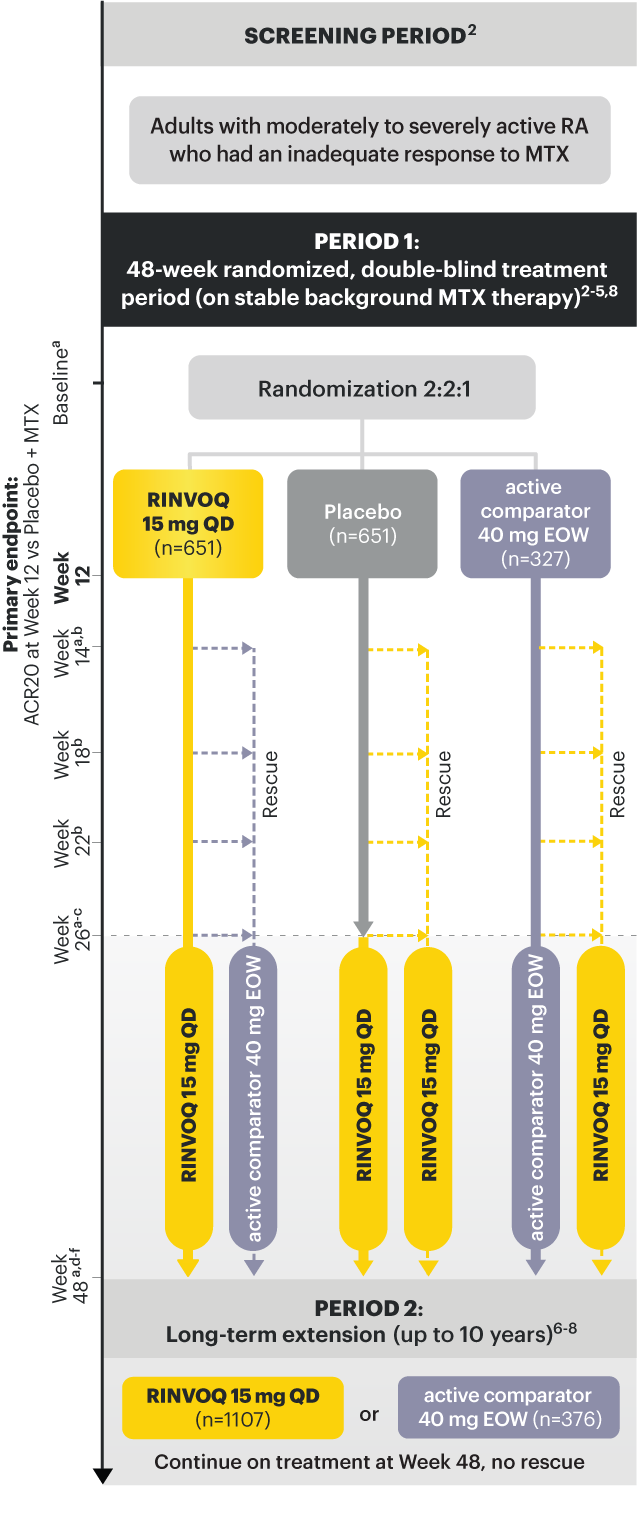
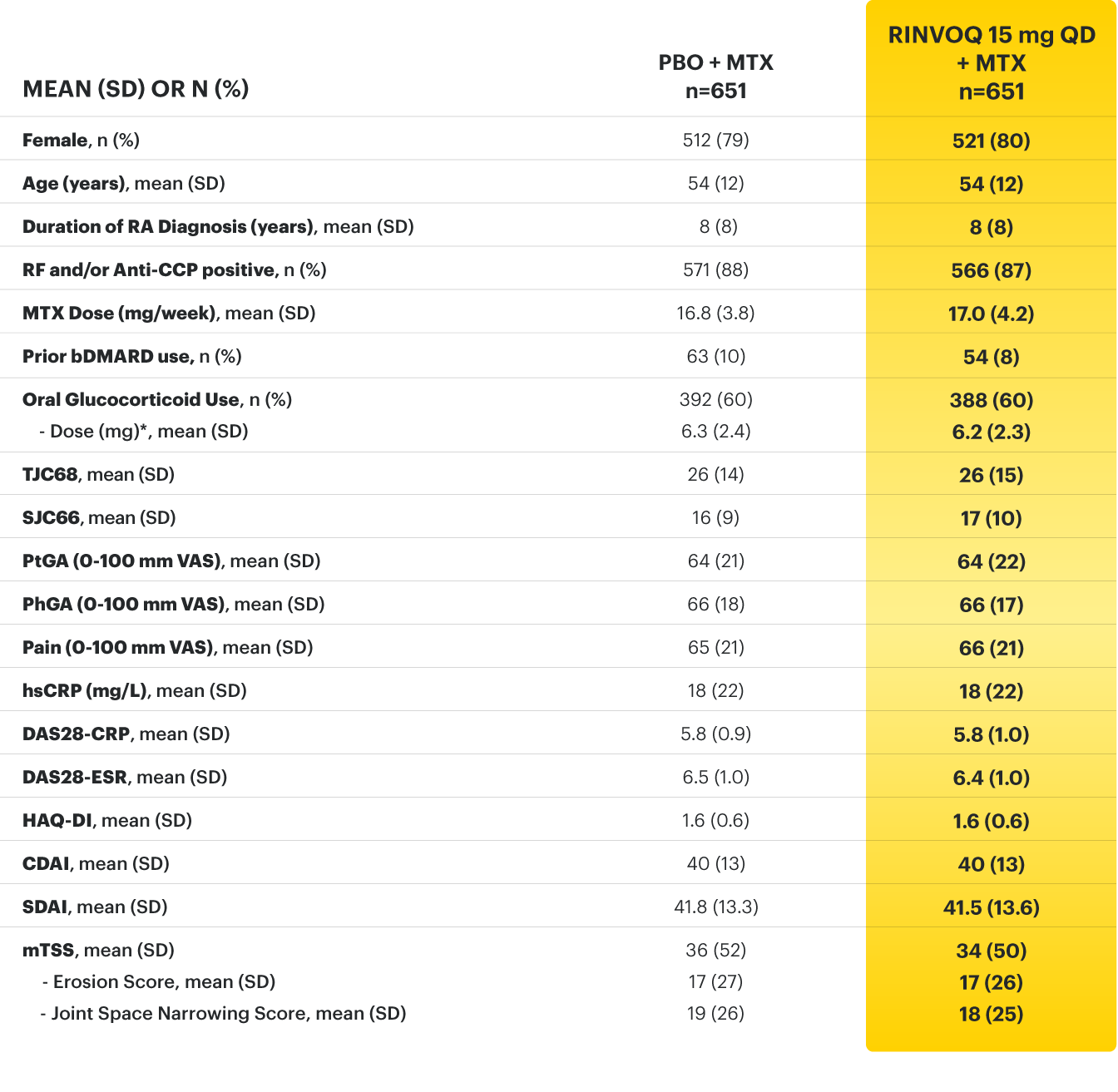
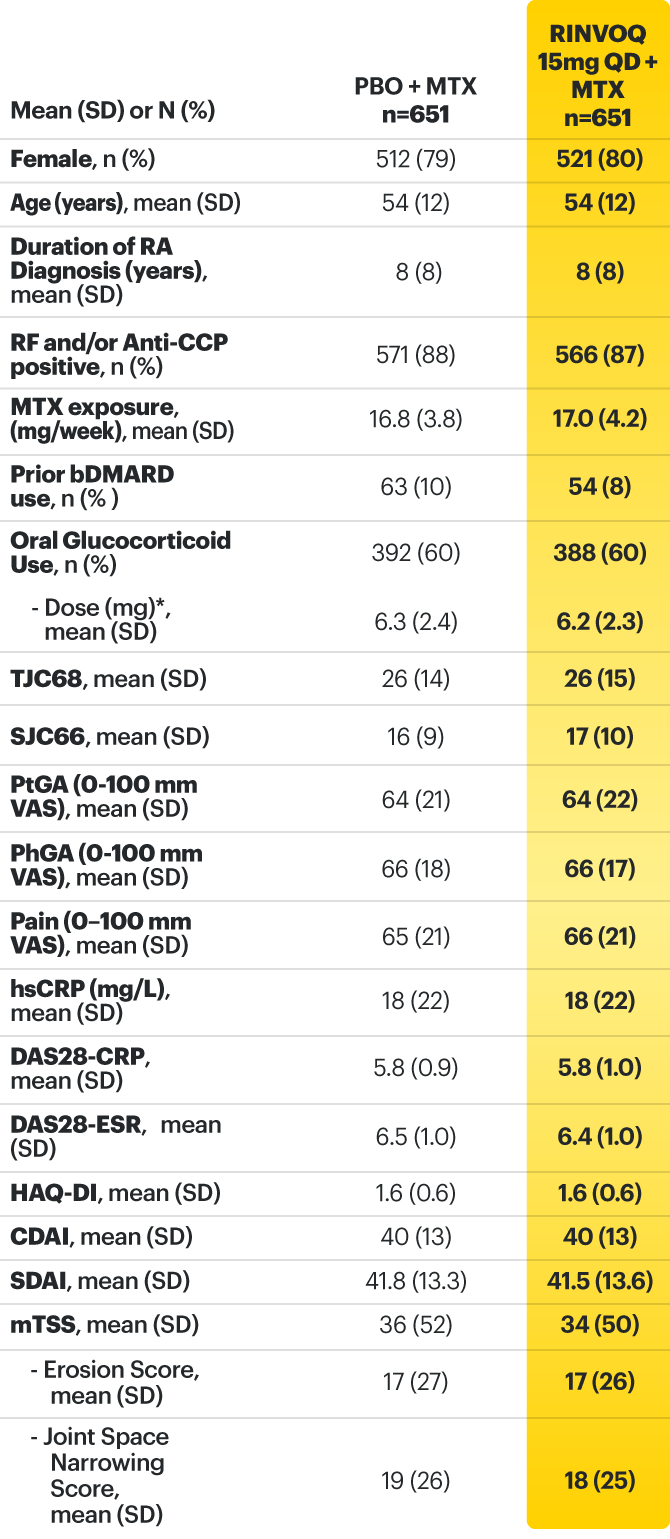
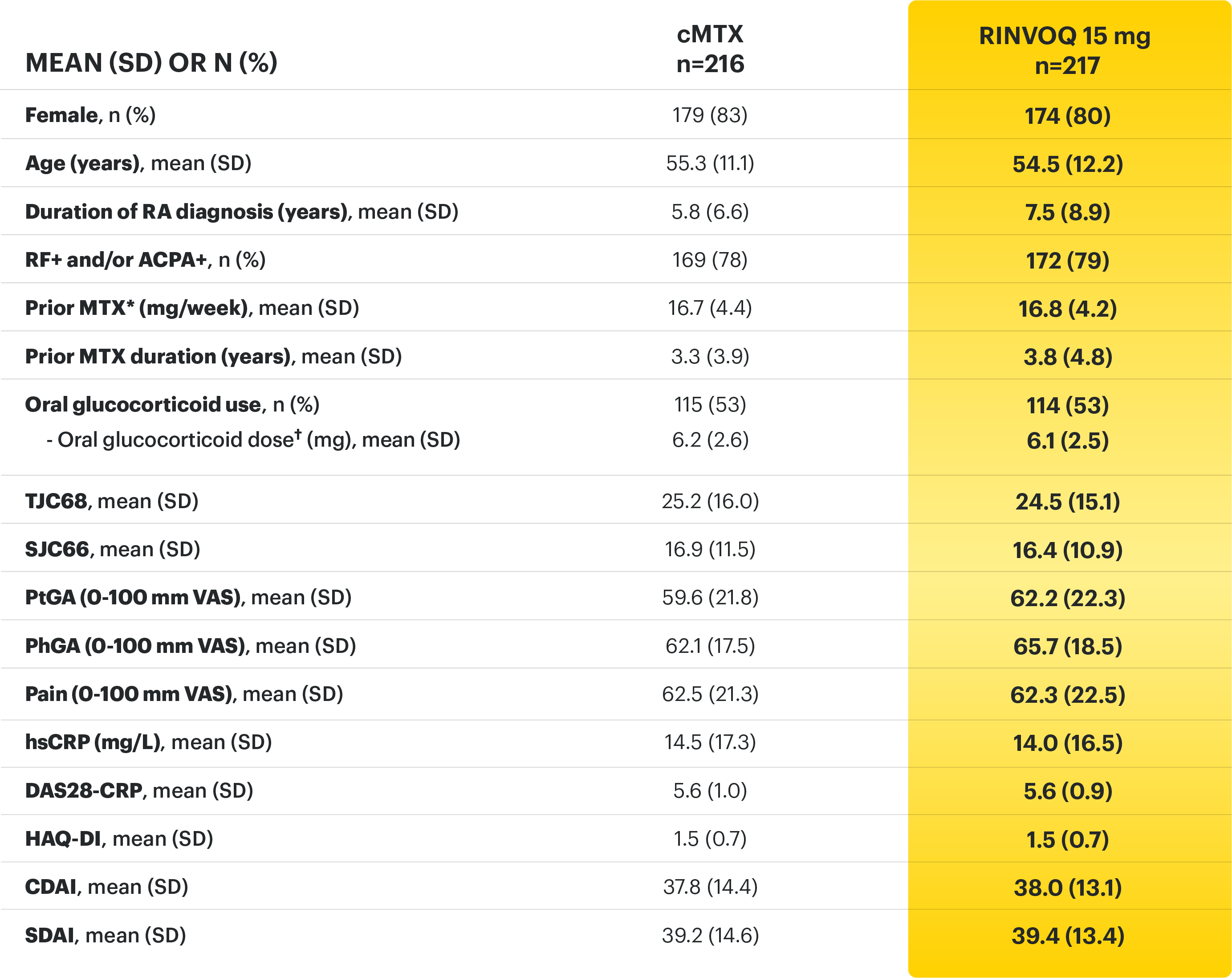
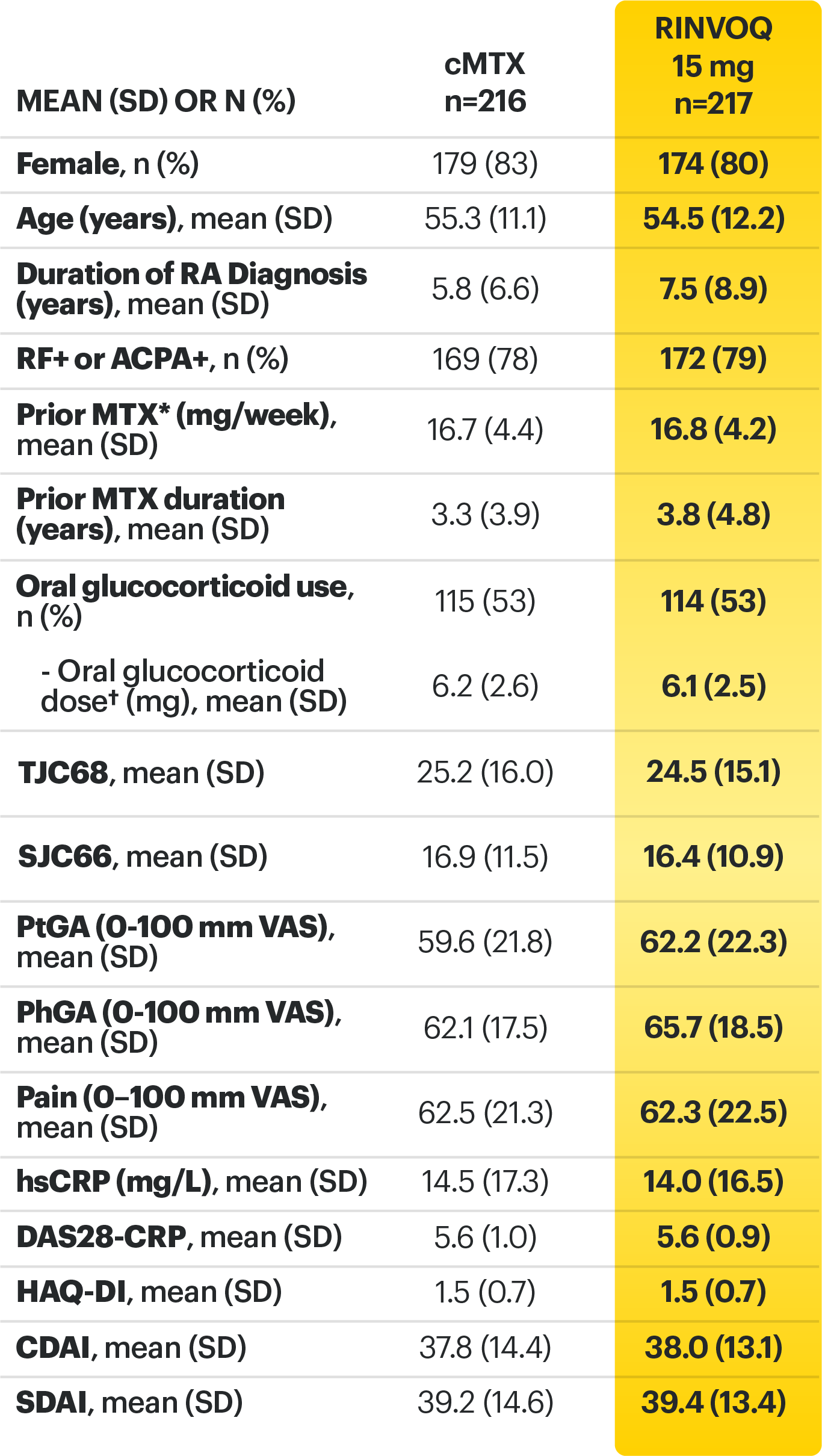
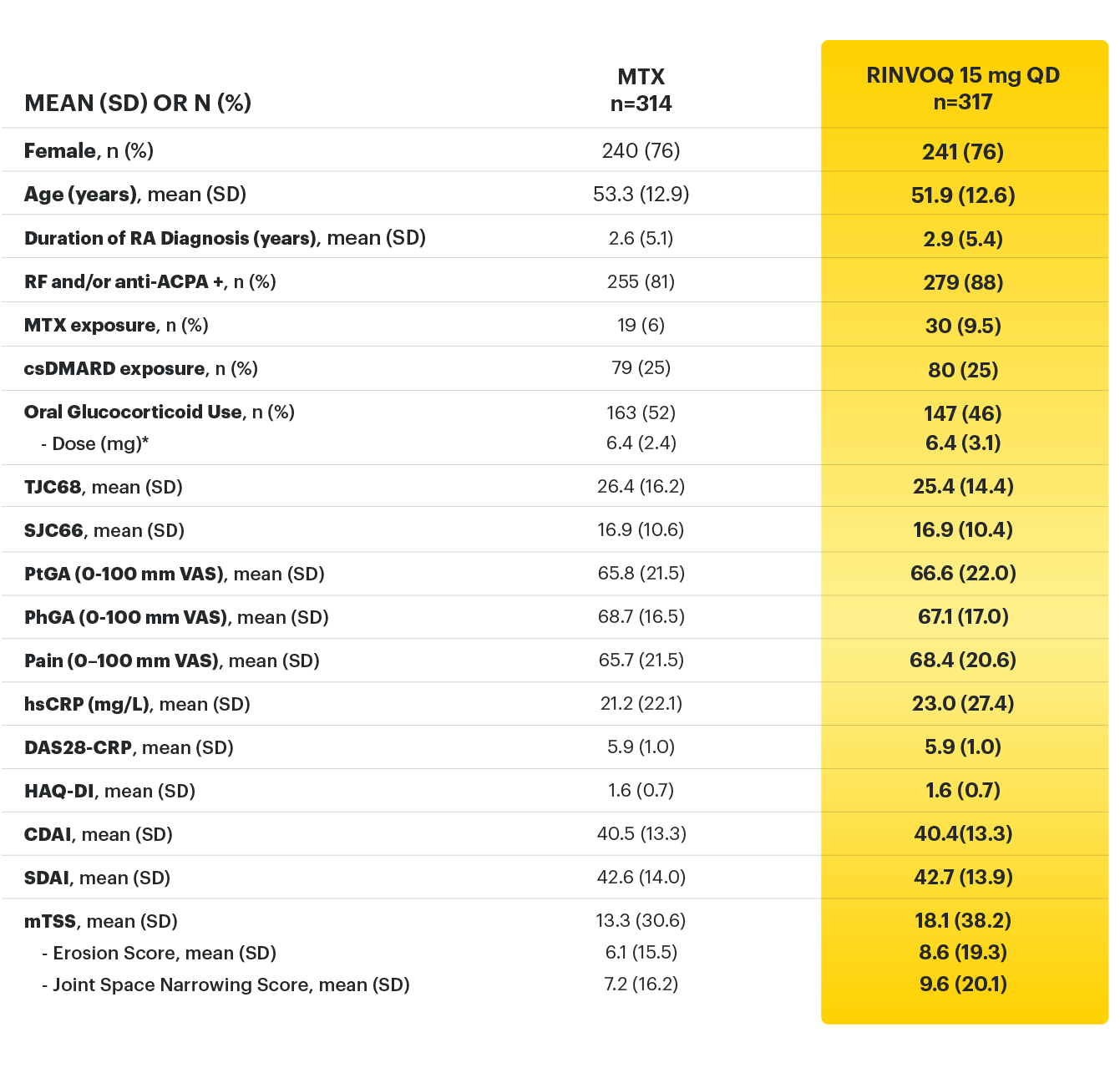
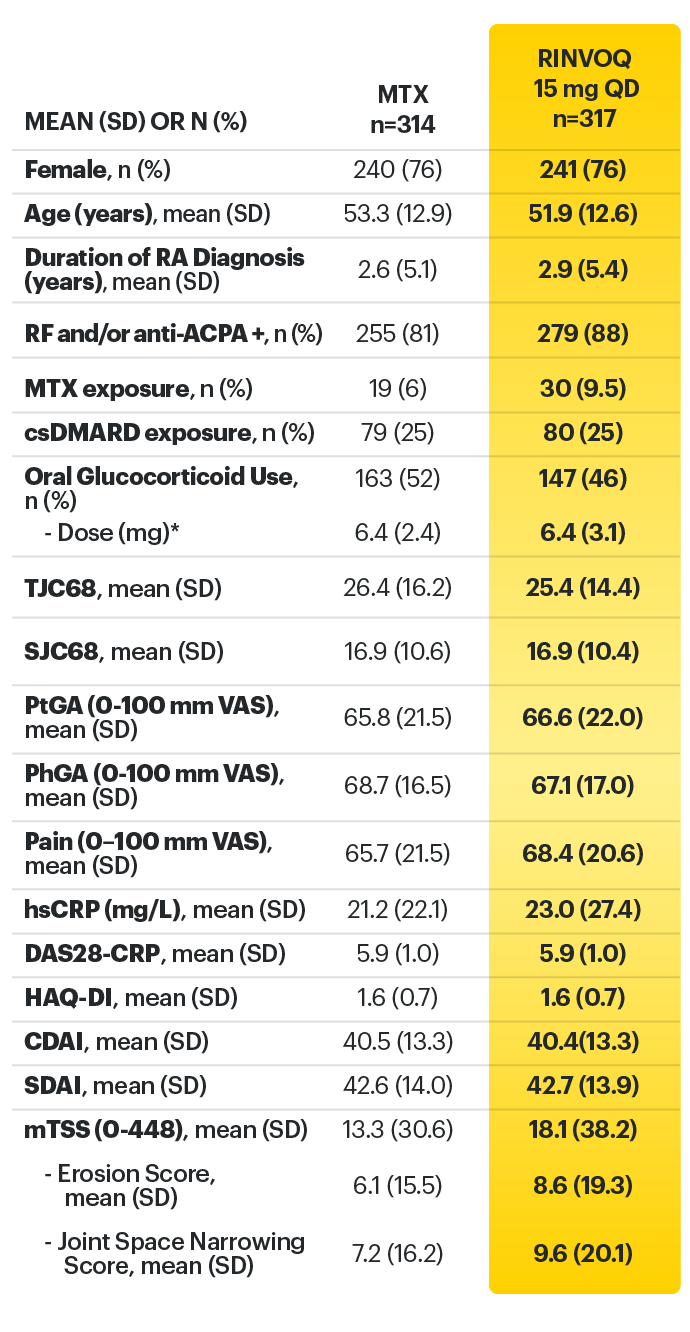
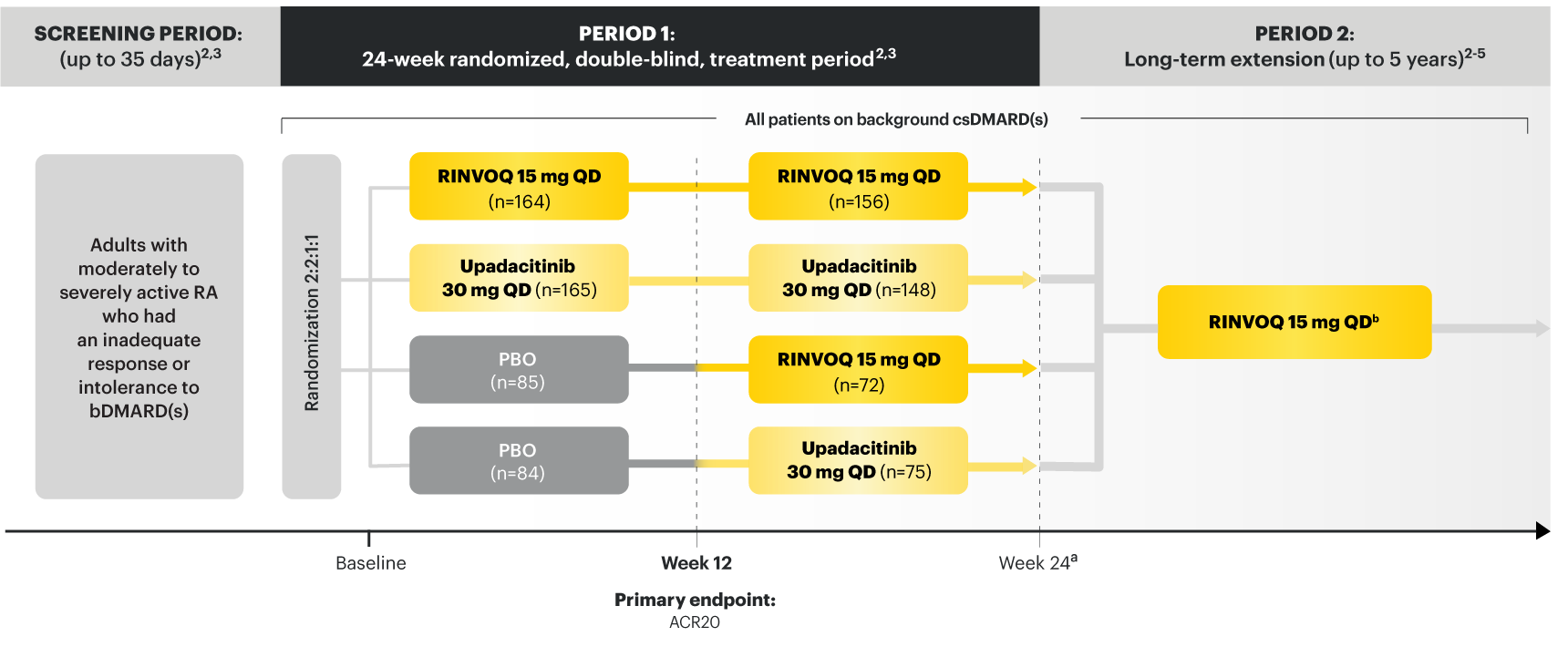
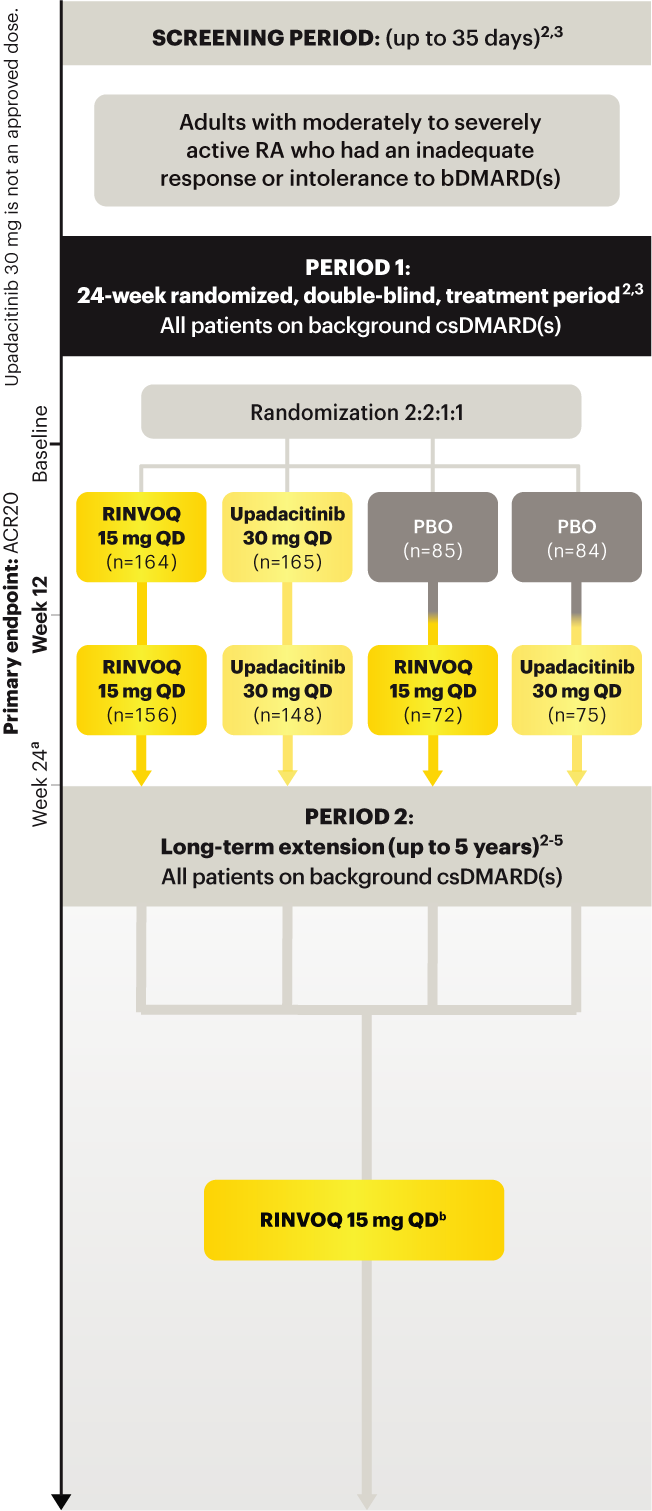
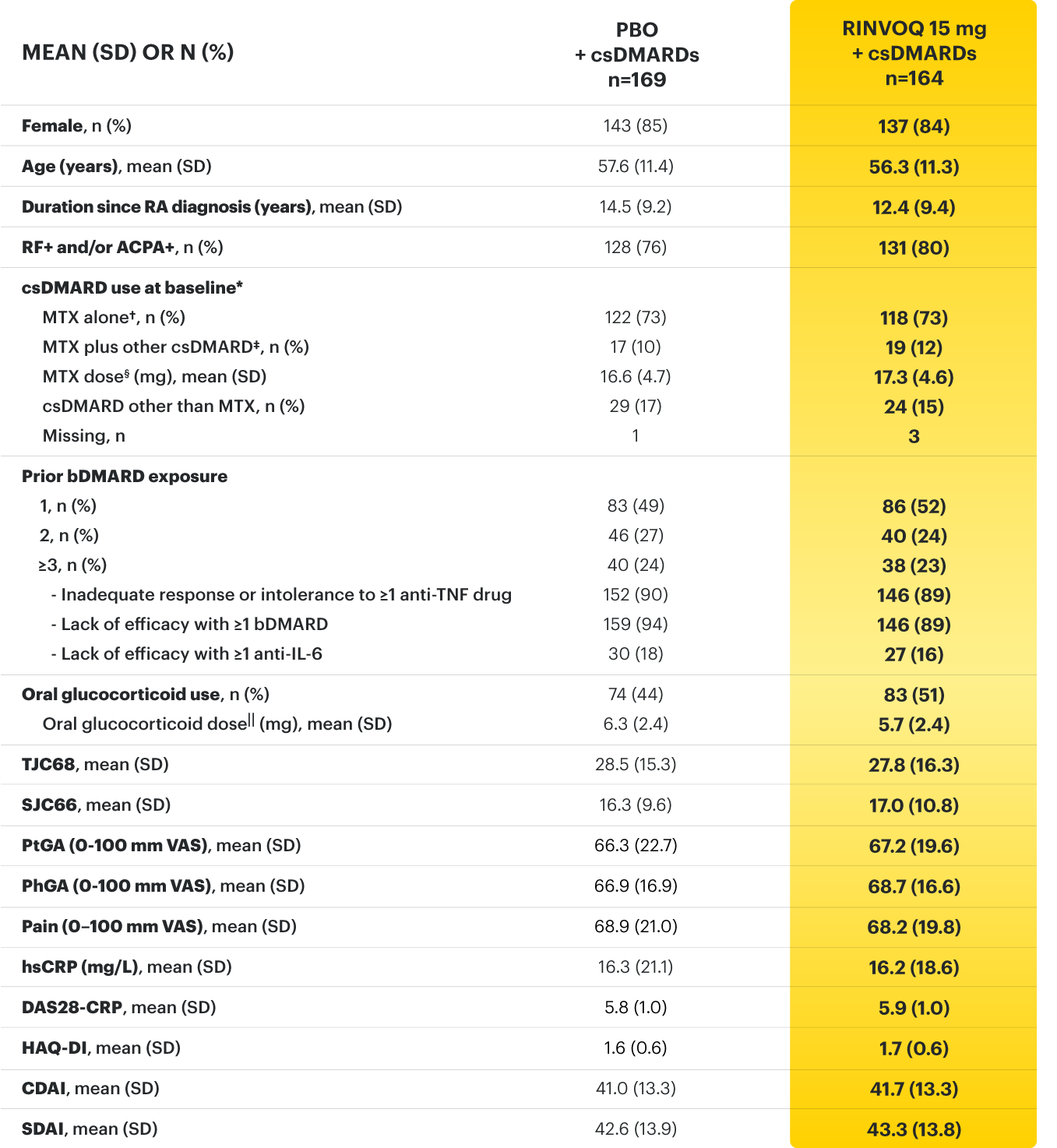
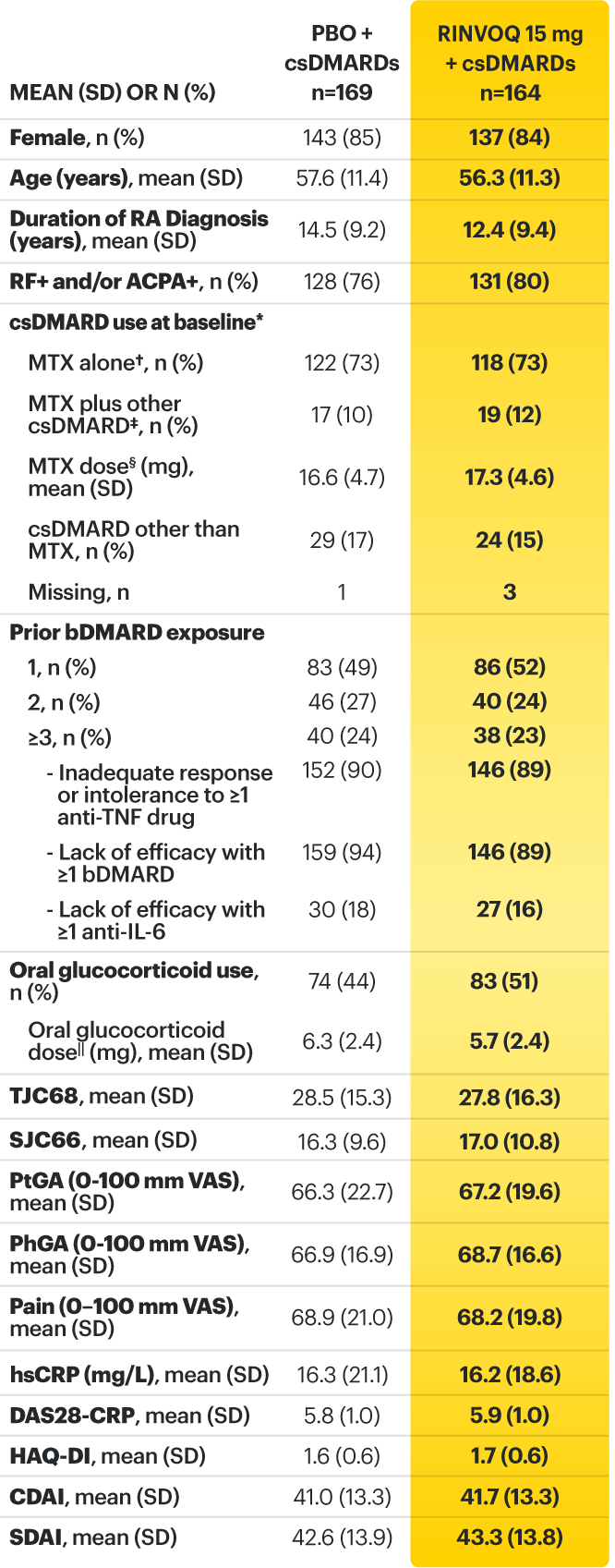
RINVOQ (upadacitinib) met all primary and ranked secondary endpoints1,7
Primary Endpoint Results
(Week 12 or 14)
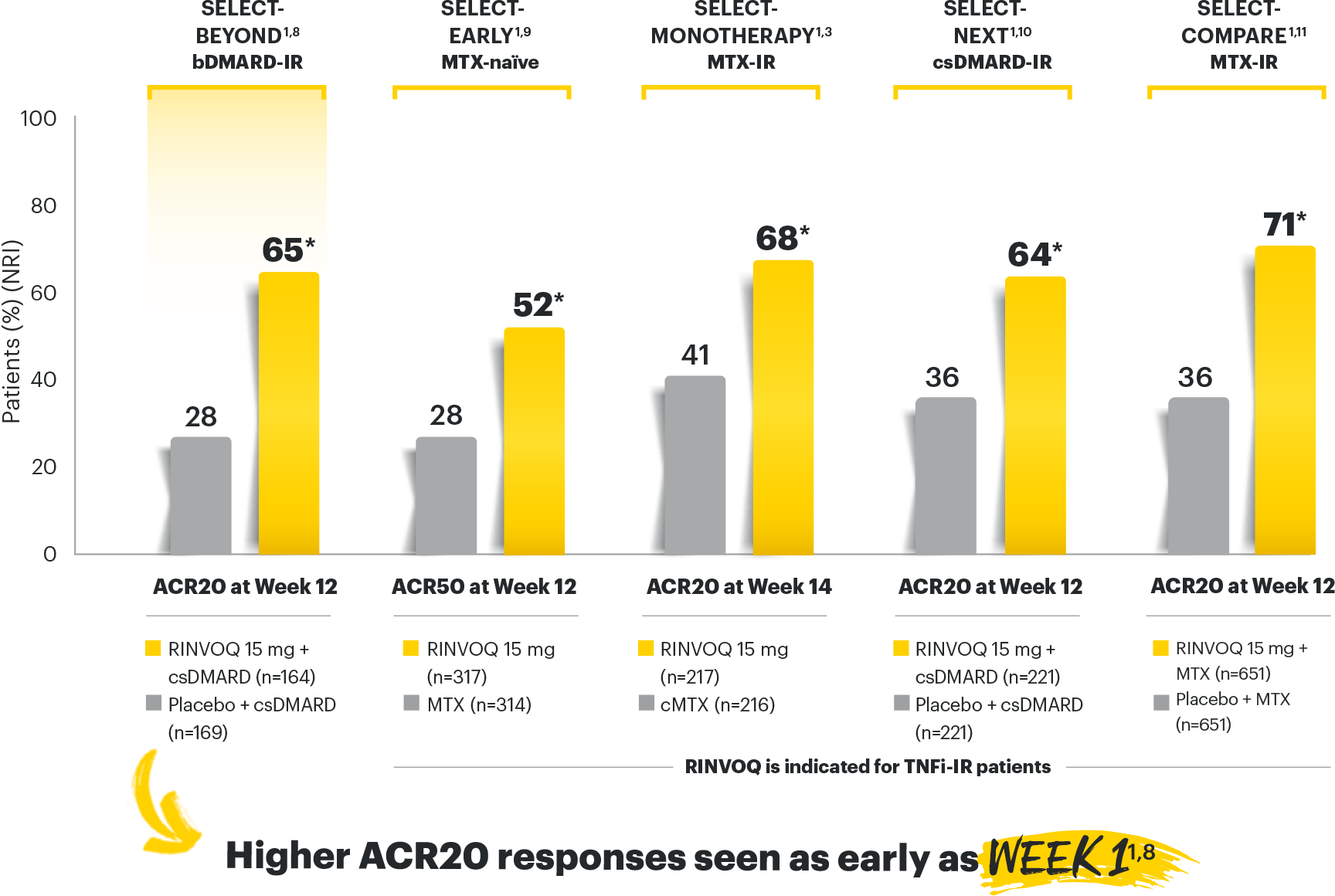

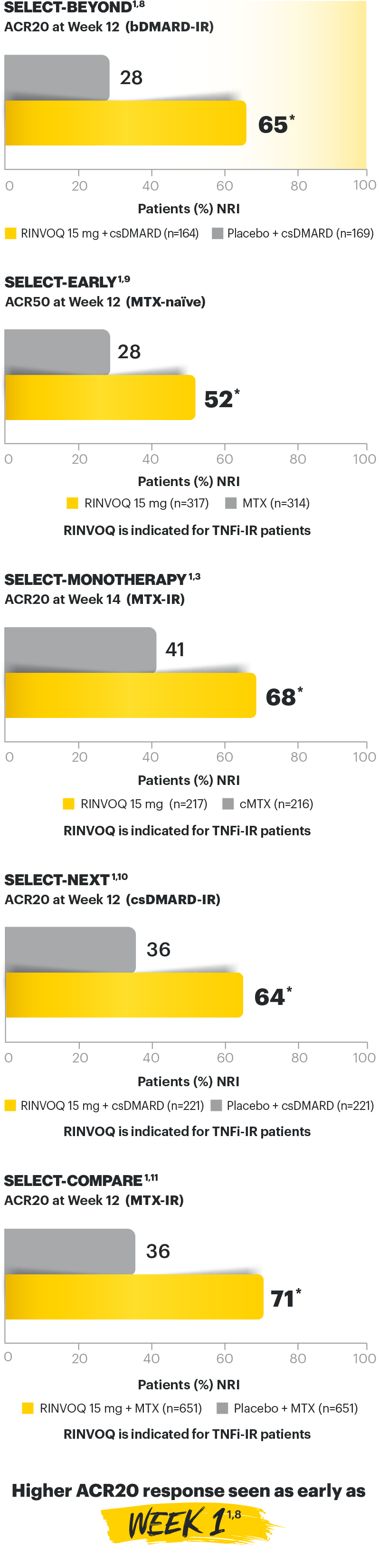
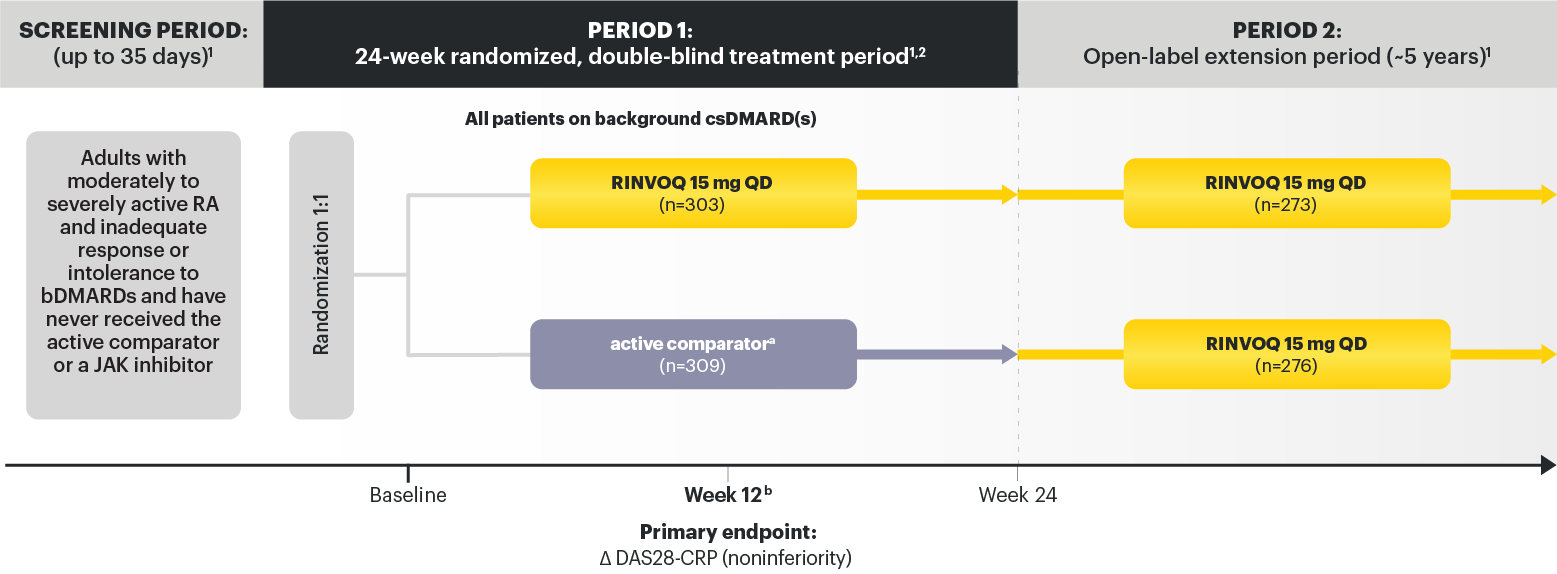
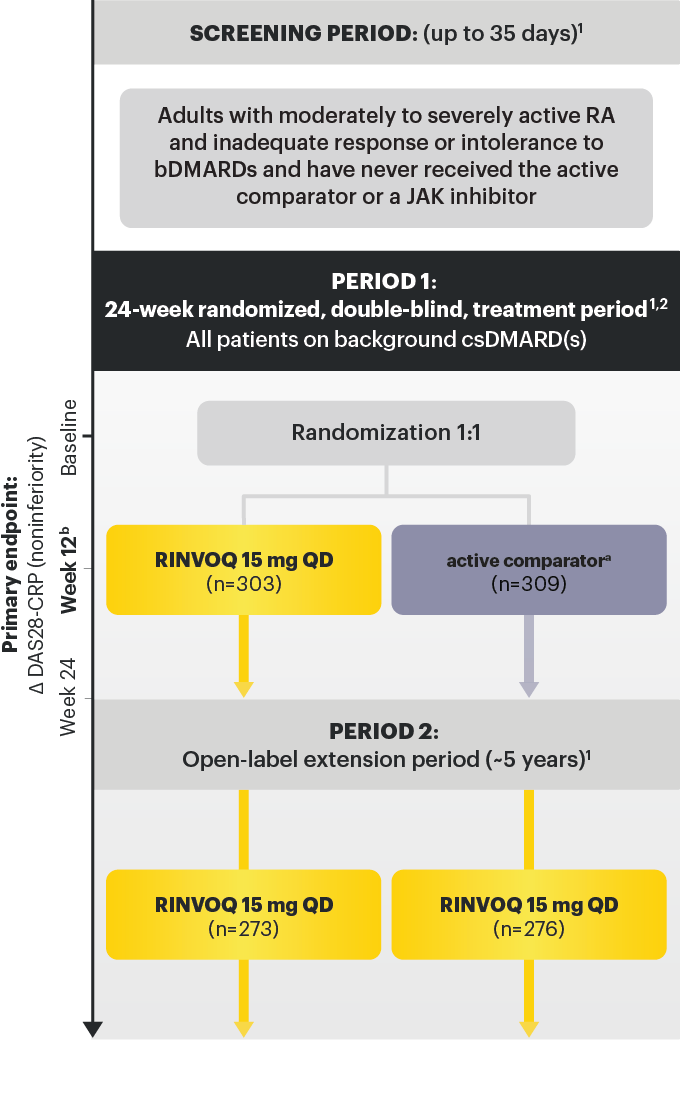
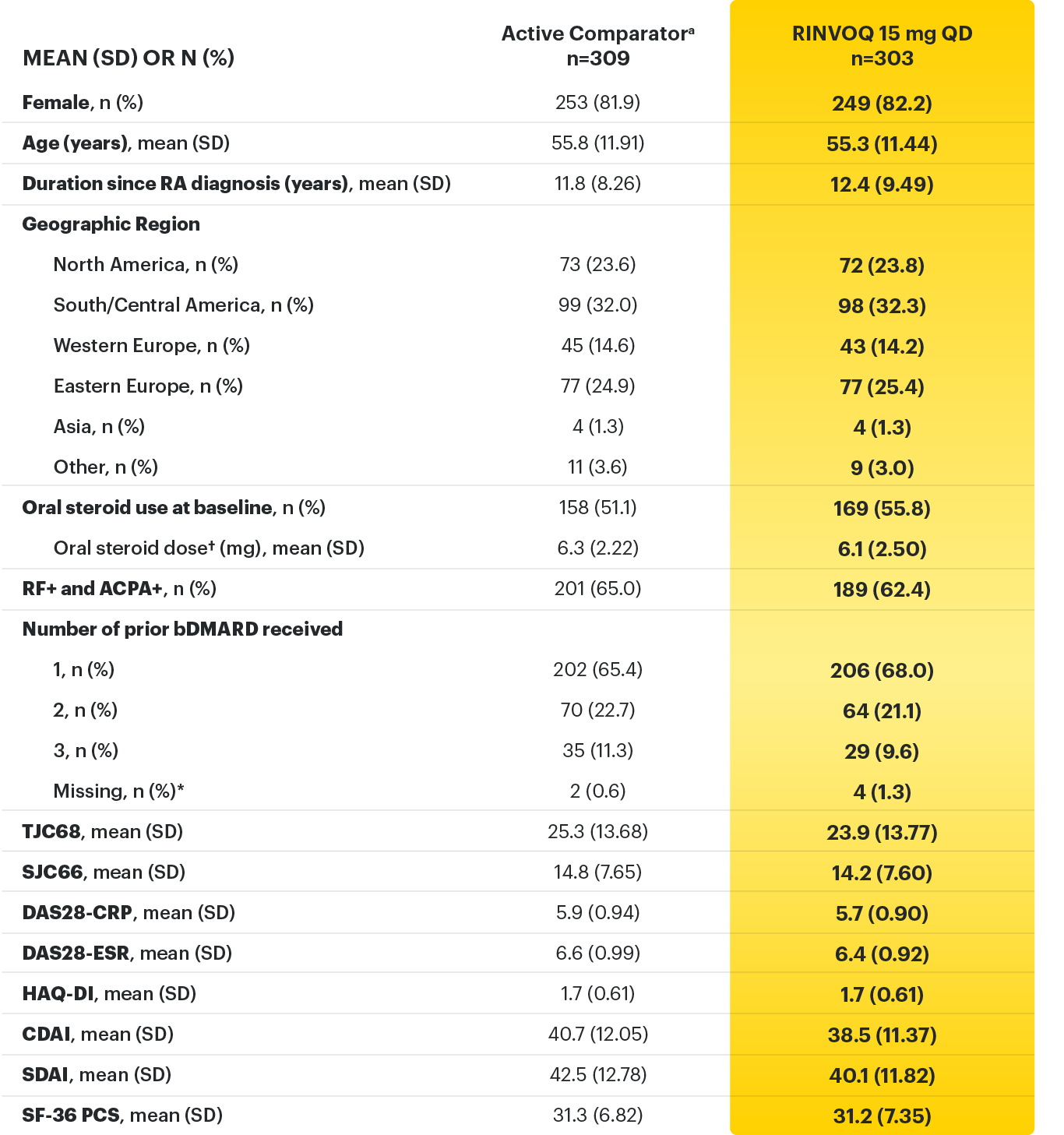
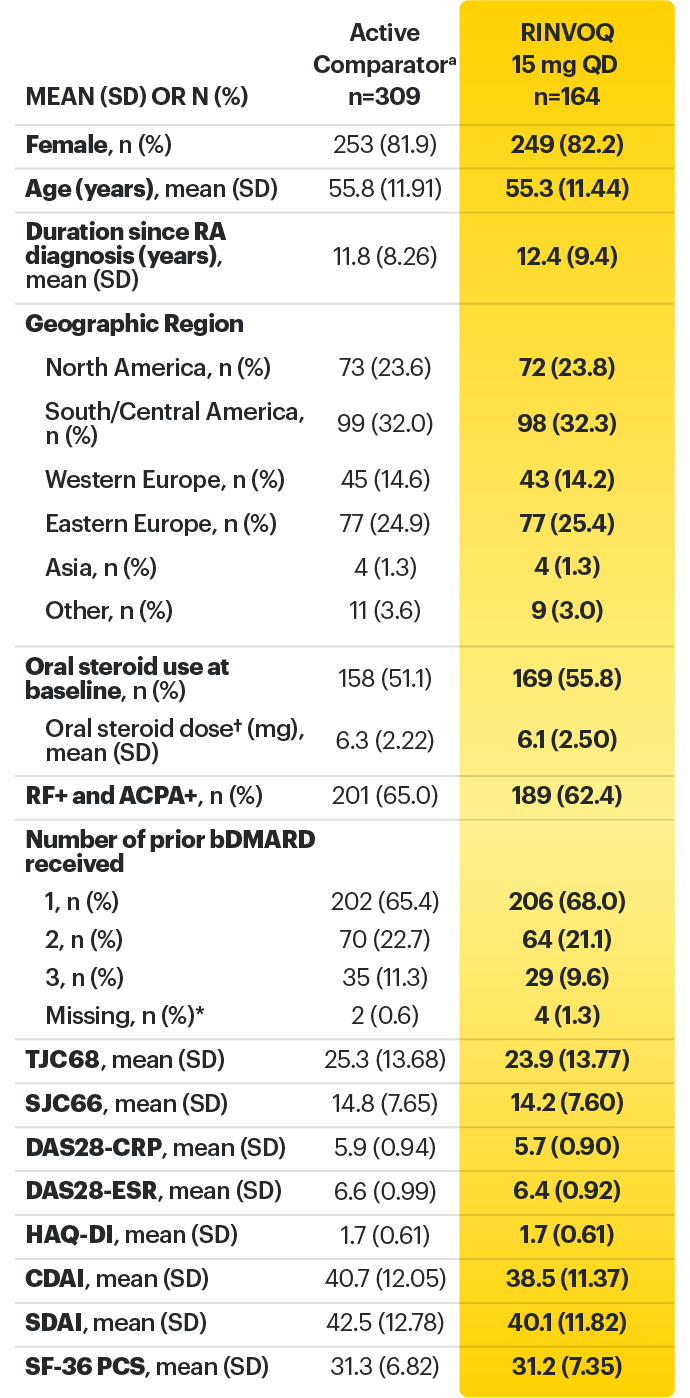
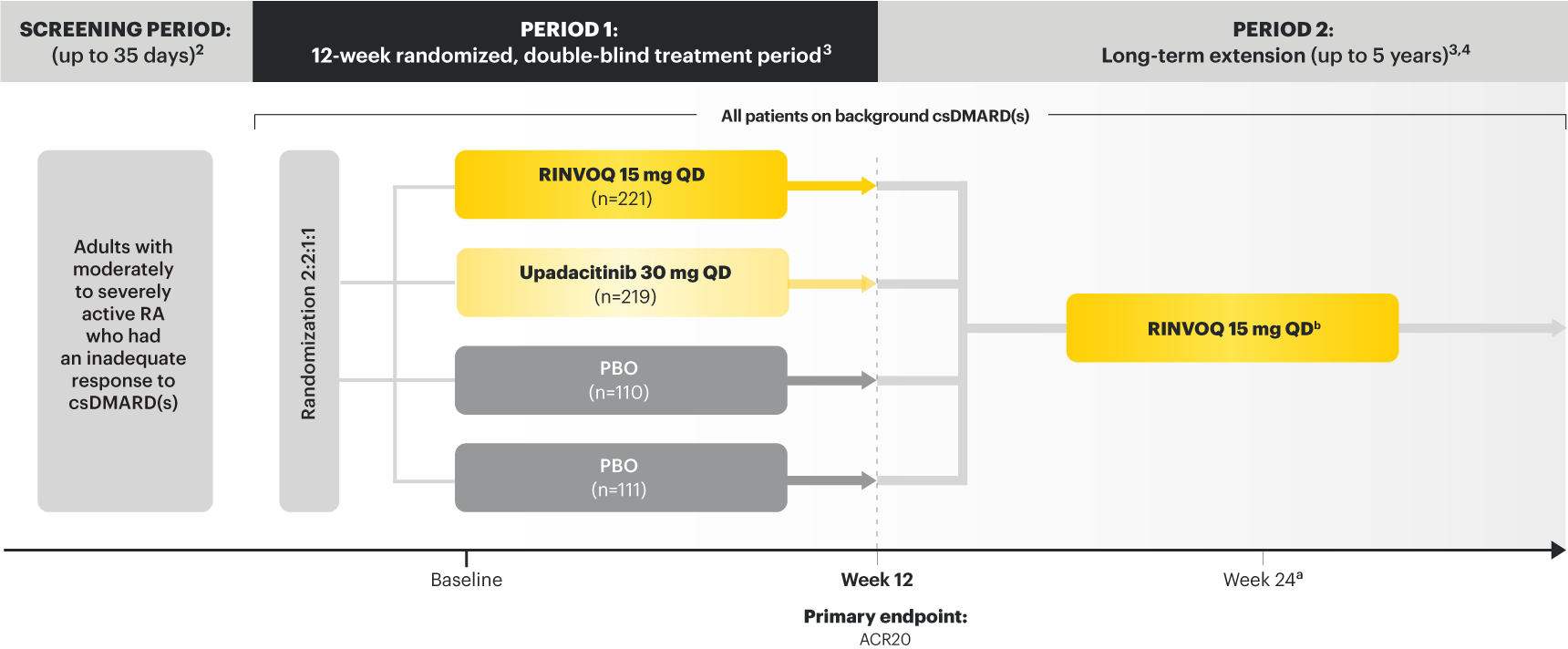
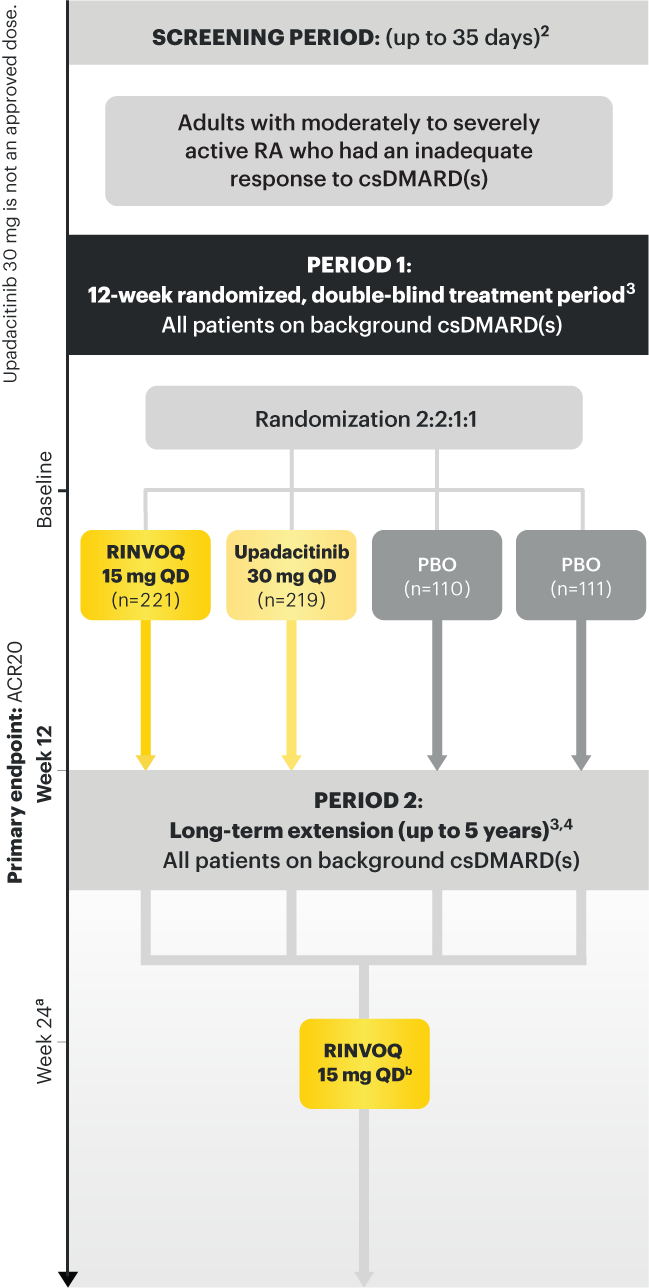
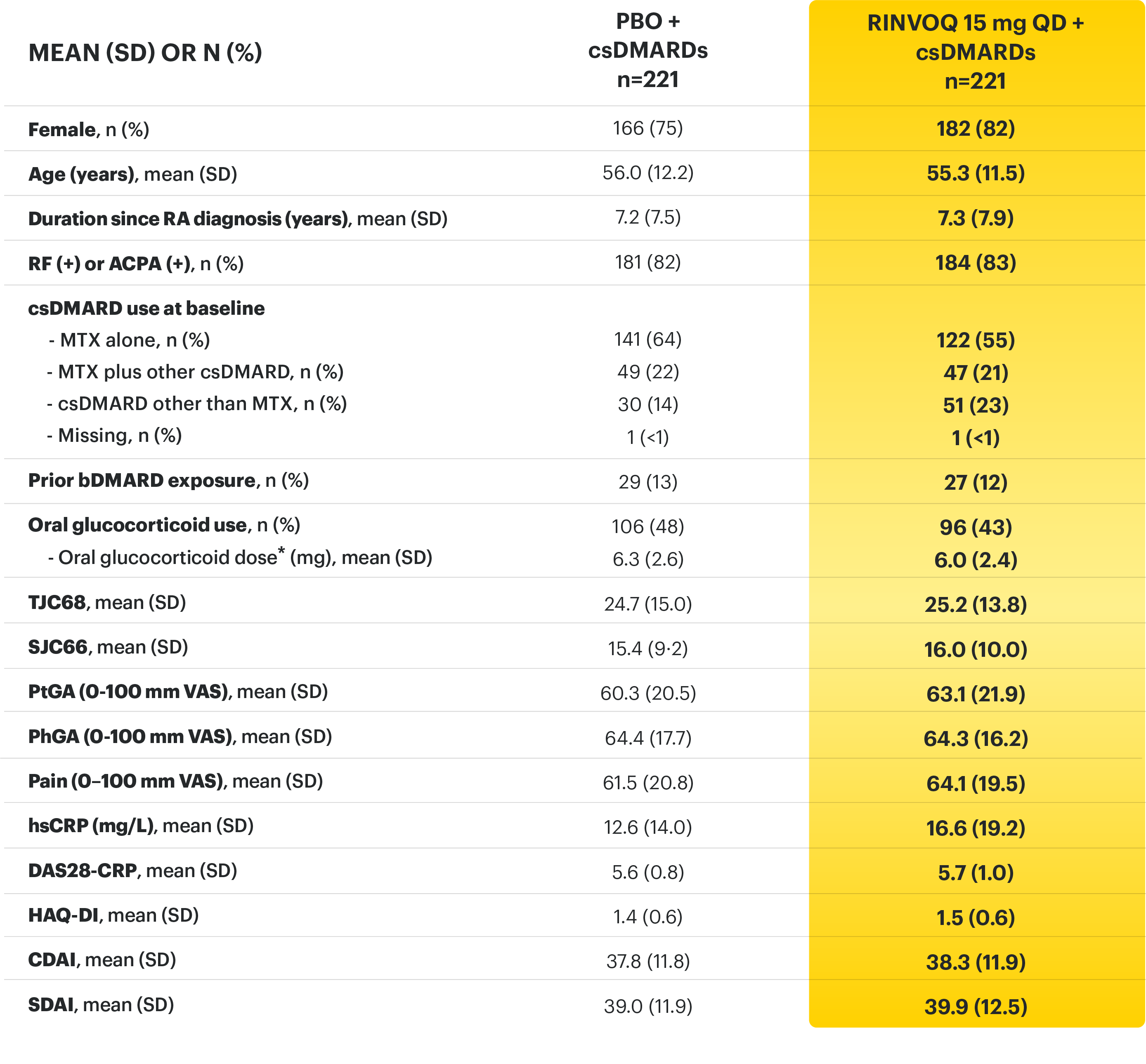
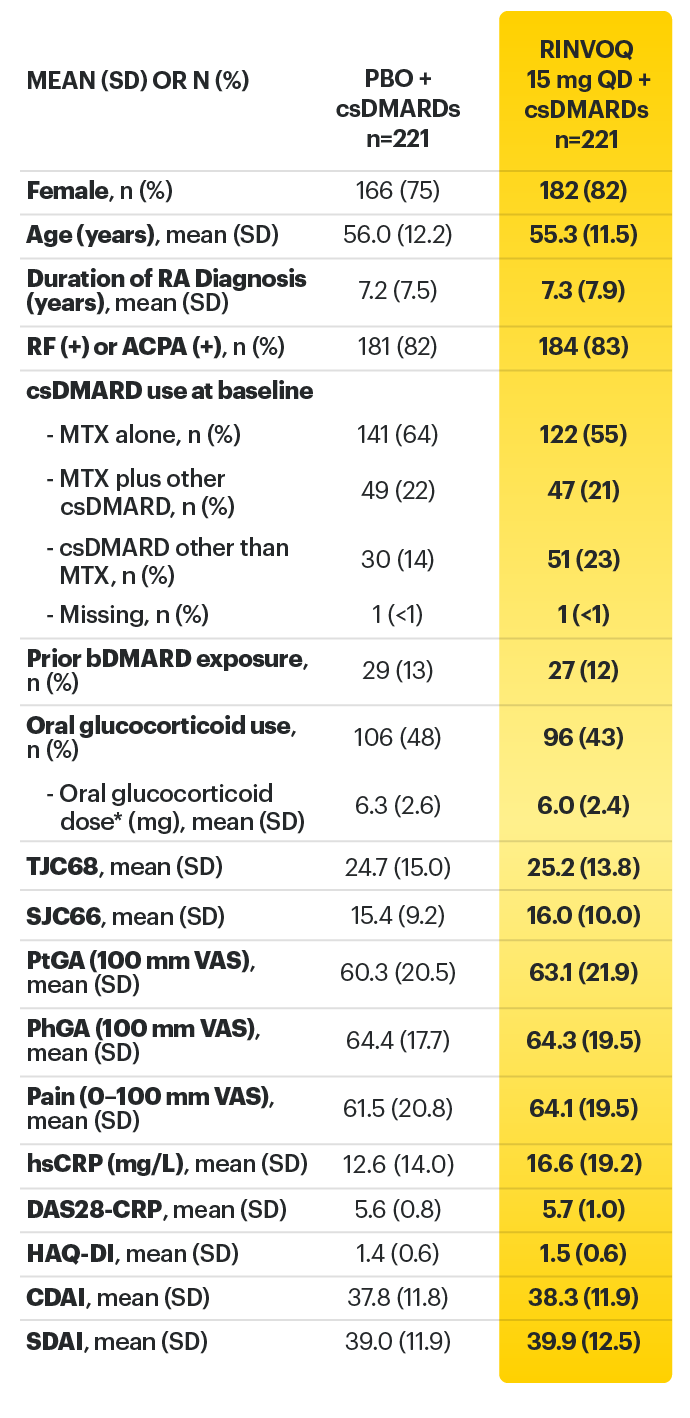
SELECT-BEYOND: 27% RINVOQ 15 mg + csDMARD vs 11% Placebo + csDMARD1,8
SELECT‑BEYOND
(Study RA‑V):1,8
12‑week, randomized, double‑blind, placebo‑controlled study of 499 adult patients with moderate to severe RA who had an inadequate response or intolerance to bDMARDs.
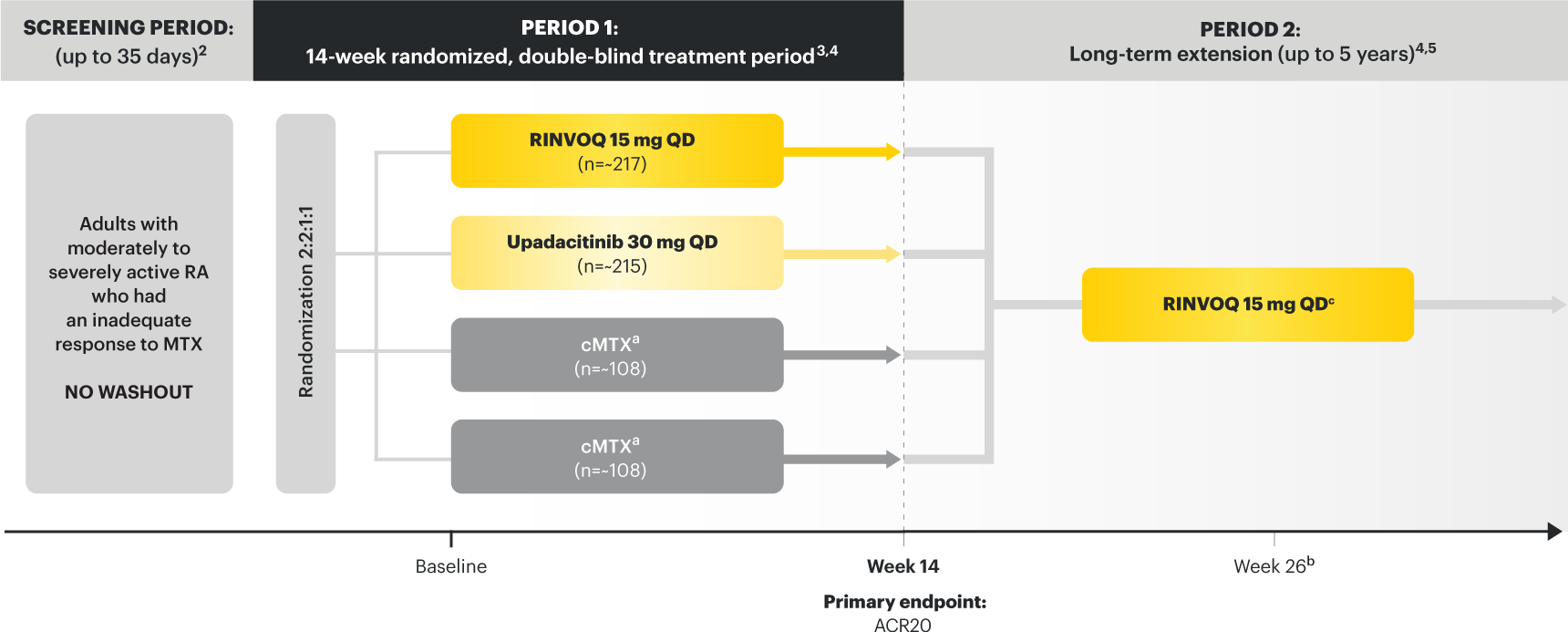
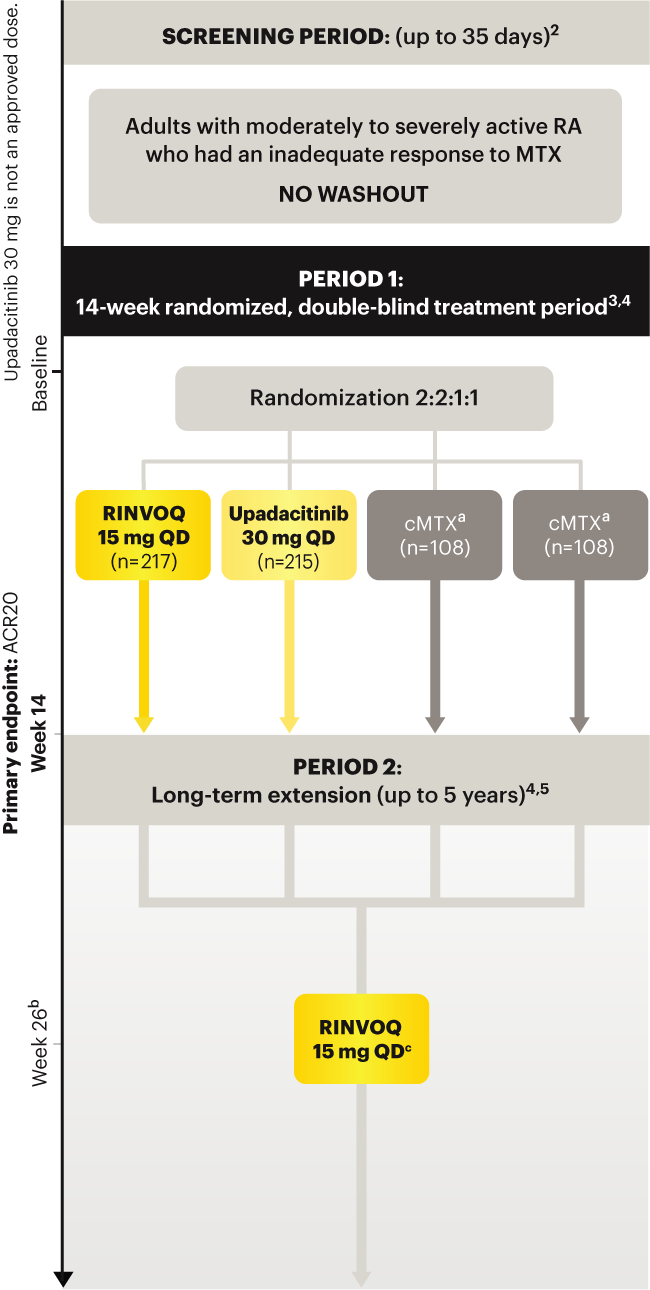
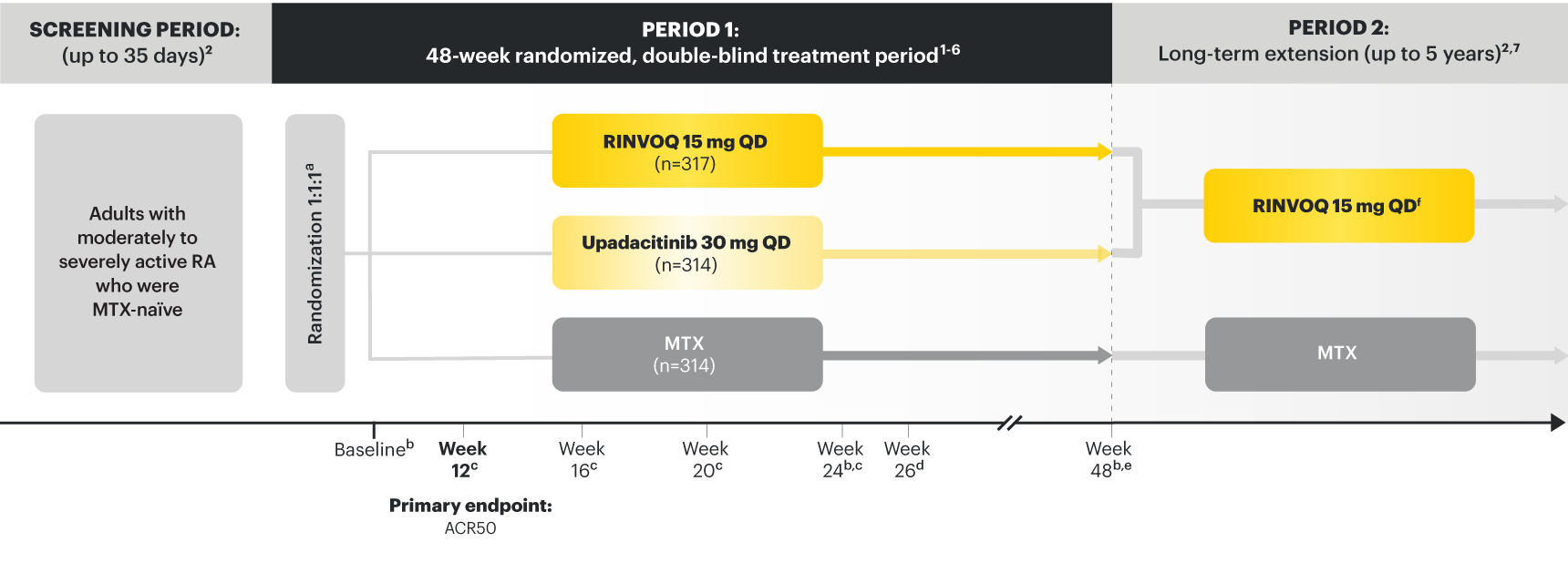
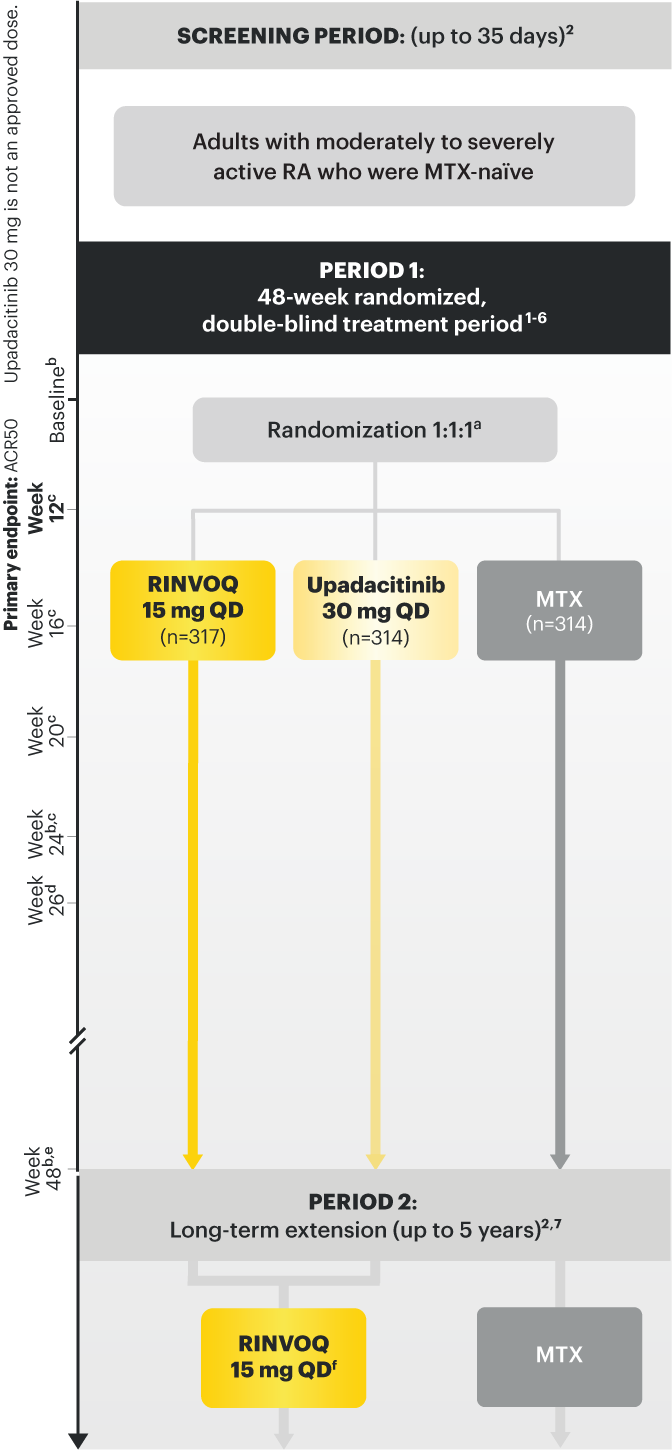
SELECT‑EARLY
(Study RA‑I):1,9
48‑week, randomized, double‑blind, active comparator‑controlled study of 947 adult patients with moderate to severe RA who were MTX‑naïve.
SELECT‑
MONOTHERAPY (Study RA‑II):1,3
14‑week, randomized, double‑blind, active comparator‑controlled study of 648 adult patients with moderate to severe RA who had an inadequate response to MTX.
SELECT‑NEXT
(STUDY RA‑III)1,10
12‑week, randomized, double‑blind, placebo‑controlled study of 661 adult patients with moderate to severe RA who had an inadequate response to csDMARDs.
SELECT‑COMPARE
(Study RA‑IV):1,11
48‑week, randomized, double‑blind, active comparator‑controlled study of 1629 adult patients with moderate to severe RA who had an inadequate response to MTX.

CONTROL THAT'S FAST AND SHOWN TO LAST
RA patients met ACR20 at Week 12 or 14 (Primary Endpoints) and Disease Control through Remission
(DAS28-CRP <2.6)* at Weeks 12 or 14 and observed up to 5 years.1,3-6
Rapid Remission1,4
- Nearly 1/3 of bDMARD-IR patients achieved remission as early as Week 12 (29% RINVOQ 15 mg + csDMARDs (n=164) vs 9% PBO + csDMARDs (n=169) in SELECT-BEYOND)†
Durable Control5,6
- Remission rates out to 5 years with or without MTX
Well-Studied Safety1,12
- >7.5 years max. exposure in RA
(~4.2 years median) to RINVOQ 15 mg as of 8/15/23‡
*Clinical remission does not mean drug-free remission or complete absence of disease activity
Exceptional Access and Patient Support2
- >95% preferred national commercial and Medicare Part D formulary coverage under the pharmacy benefit as of January 20242,§,‖
- 1:1 support to help RA patients start and stay on track with their prescribed treatment plan
†P<0.001. Analyses were not controlled for multiplicity. P-values obtained through nominal statistical testing.
‡In PsA: ~5.7 years maximum exposure (~3.7 years median) to RINVOQ 15 mg as of 08/2023. In AS: ~3.8 years maximum exposure (~1.7 years median) to RINVOQ 15 mg as of 08/2023. In nr‑axSpA: ~3.4 years maximum exposure (~1 years median) to RINVOQ 15 mg as of 08/2023.12
§RINVOQ is on a preferred tier or otherwise has preferred status on the plan's formulary.
‖Coverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biological disease-modifying antirheumatic drug; CRP=C-reactive protein; csDMARDs=conventional synthetic disease modifying antirheumatic drugs; DAS28-CRP=28 joint disease activity score using C-reactive protein; IR=intolerance or inadequate response; max.=maximum; MTX=methotrexate; PBO=placebo; RA=rheumatoid arthritis; TNFi=tumor necrosis factor inhibitor