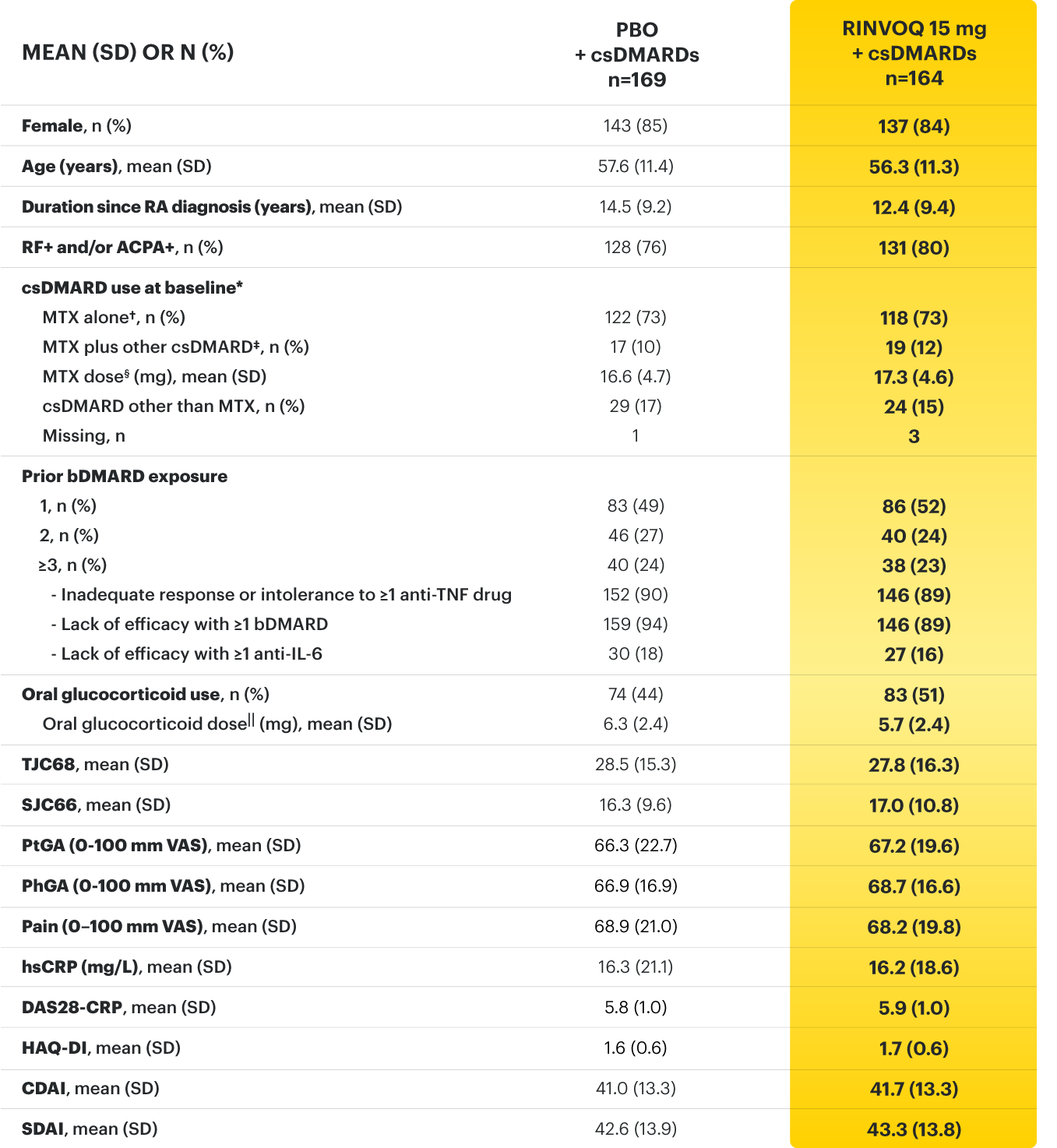
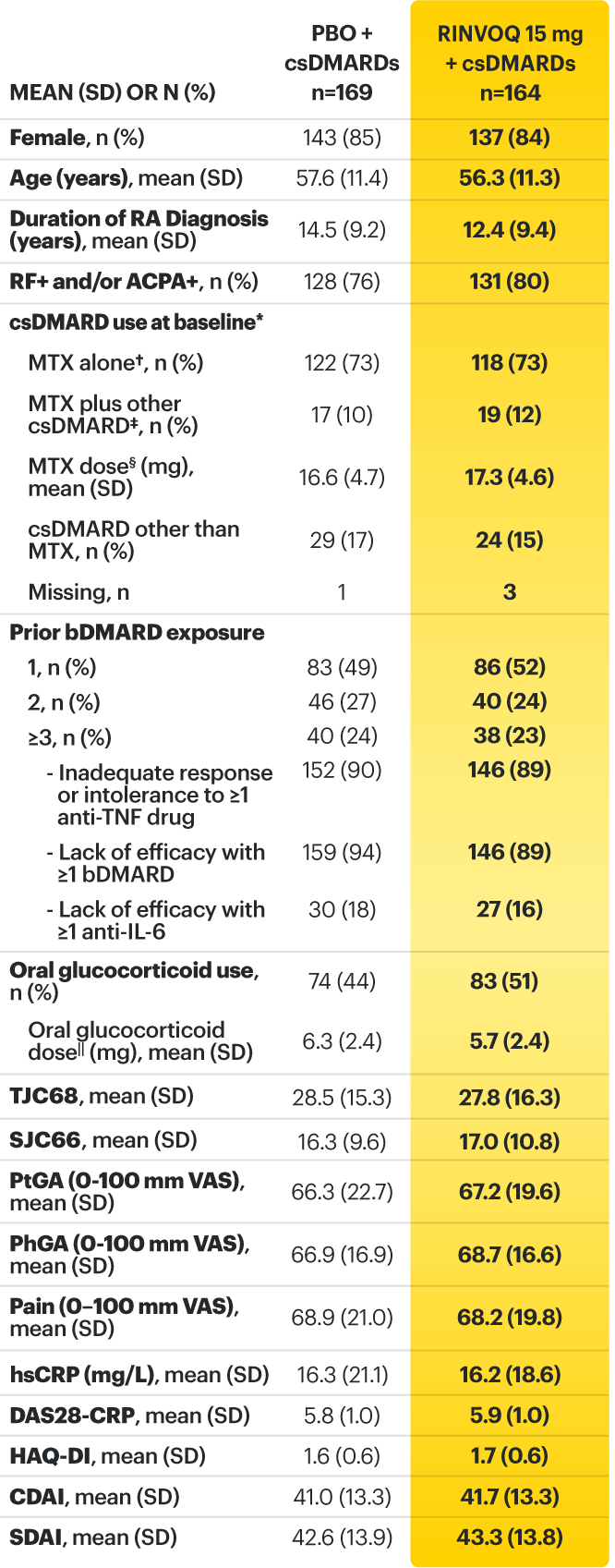
For moderate to severe rheumatoid arthritis (RA) in adult TNFi-IR patients1
IR=intolerance or inadequate response; TNFi=tumor necrosis factor inhibitor
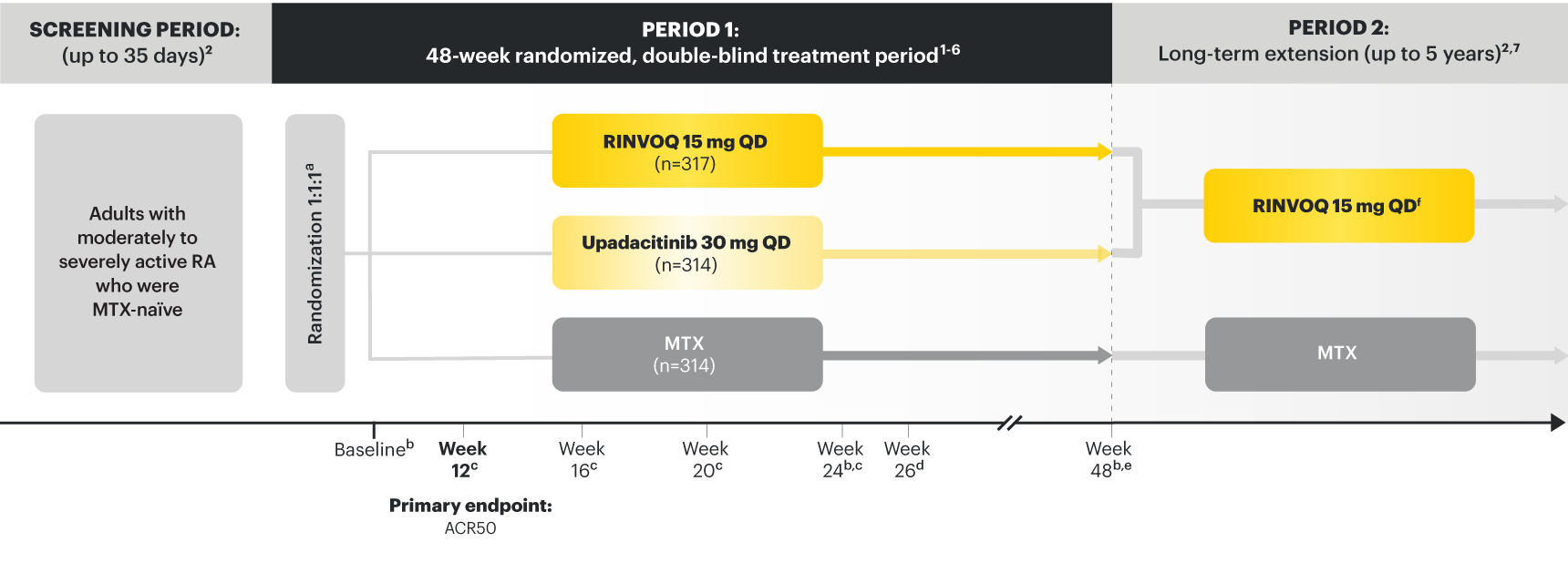
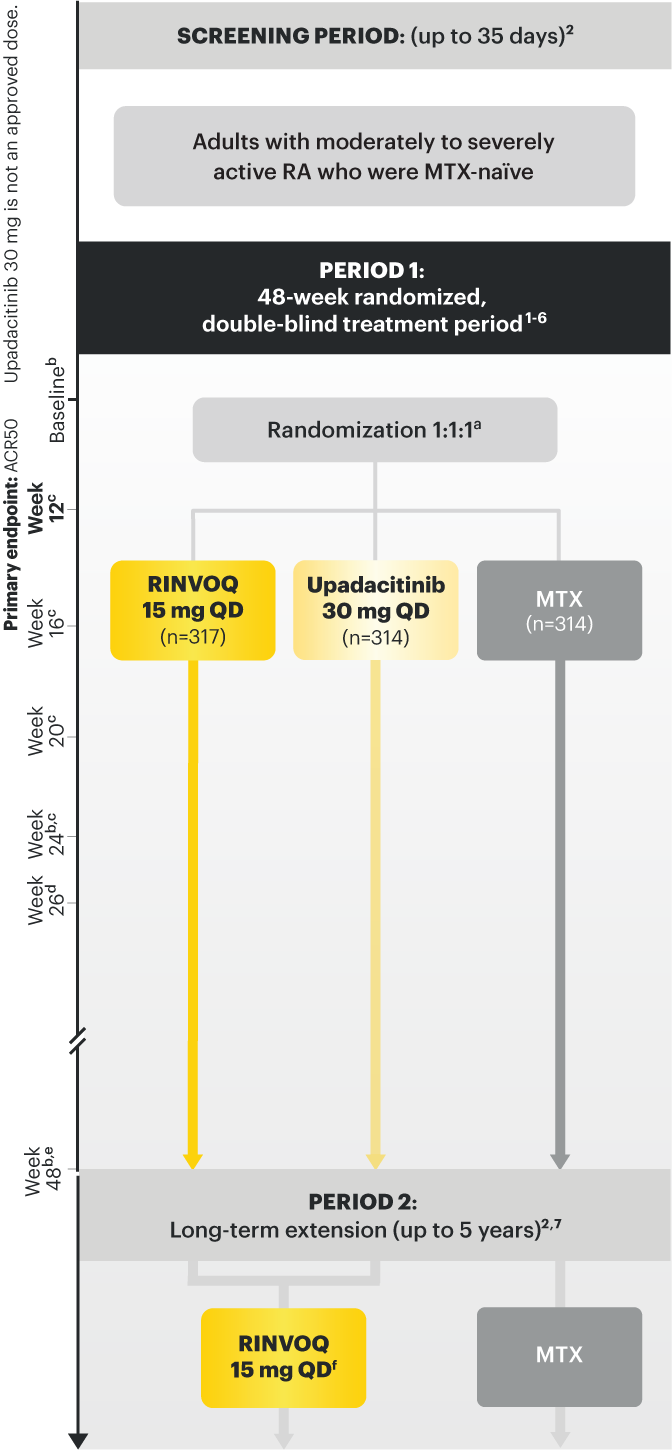
A ONCE-DAILY ORAL THERAPY
STUDIED WITH OR WITHOUT MTX IN RA1
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biologic disease-modifying antirheumatic drugs (bDMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine.1
- RINVOQ is a 15 mg extended-release pill.1
- Take one pill 1 time a day with or without food.
- Advise patients to avoid food or drink containing grapefruit during treatment with RINVOQ.
- Swallow pill whole. Do not split, crush, or chew.
- Instruct patients to notify their healthcare provider if they repeatedly notice intact RINVOQ tablet or fragments in stool or ostomy output.
- Store at 36˚F to 77˚F (2˚C to 25˚C) in the original bottle in order to protect from moisture.
- In clinical trials, RINVOQ mean terminal elimination half-life ranged from 8-14 hours.




Awarded the Arthritis Foundation Ease of Use Commendation,2 our innovative bottle cap includes:
- Wide, easy-to-grip texture
- Embedded tool that seamlessly punctures the foil liner to simplify medication access

IR=intolerance or inadequate response; MTX=methotrexate; RA=rheumatoid arthritis; TNFi=tumor necrosis factor inhibitor
Lab Monitoring
and
treatment considerations1
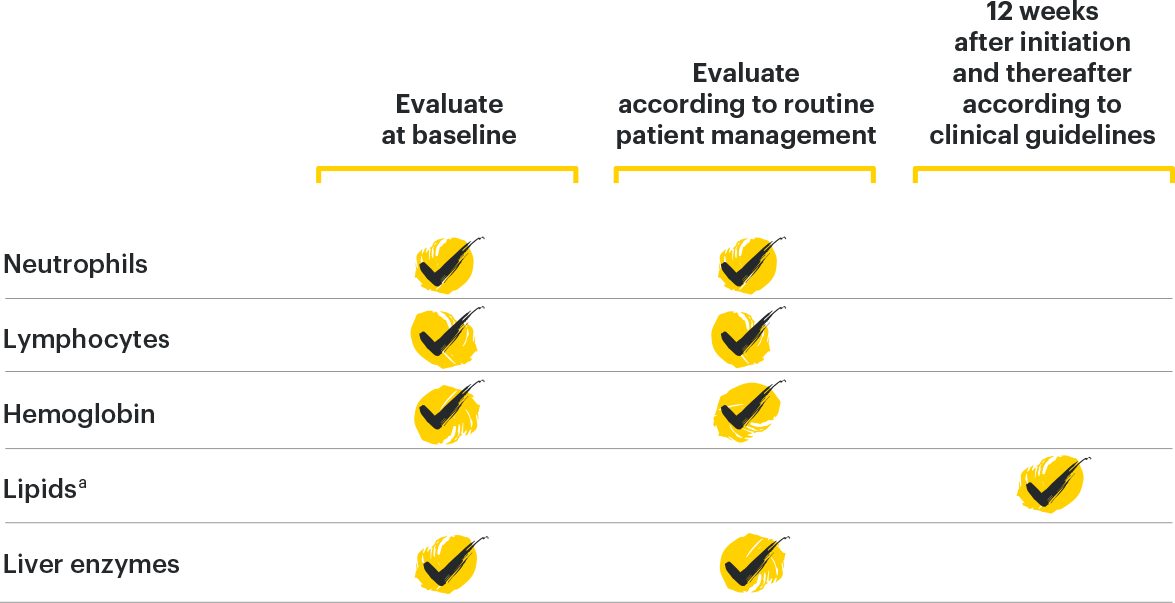

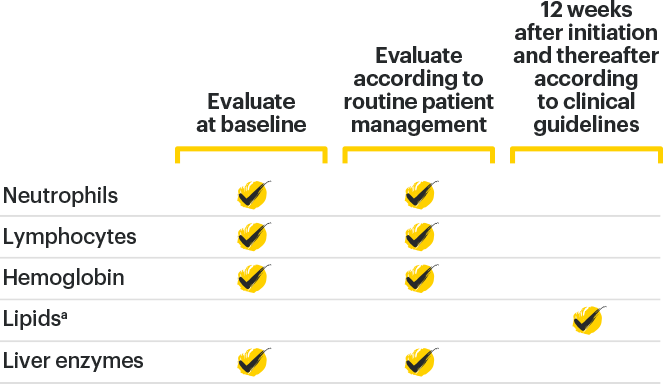
TREATMENT WITH RINVOQ SHOULD NOT BE INITIATED, OR SHOULD BE INTERRUPTED IF:
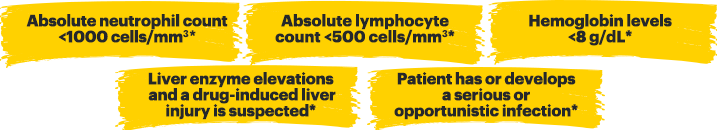


*Treatment can be initiated or restarted after levels return above specified values, drug-induced liver injury diagnosis is excluded, or infection is controlled.
In RA, PsA, AS, and nr‑axSpA:
No dose adjustment is required for mild, moderate or severe renal impairment.1
No dose adjustment is required for mild or moderate hepatic impairment.1
RINVOQ is not recommended in patients with:
Active hepatitis B or hepatitis C
Severe hepatic impairment (Child‑Pugh C)
RINVOQ has not been studied in end‑stage renal disease (eGFR <15 mL/min/1.73m2)
HYPERSENSITIVITY:1 RINVOQ is contraindicated in patients with known hypersensitivity to upadacitinib or any of its excipients. If a clinically significant hypersensitivity reaction occurs, discontinue RINVOQ and institute appropriate therapy.
Rinvoq is a JAK inhibitor1
Why JAKs are important in RA3-7
Pro-inflammatory cytokines use immune signaling networks, such as the JAK-STAT pathway, to communicate with the cell nucleus. When dysregulated, as in RA, these signals increase the inflammatory response, leading to cycles of chronic inflammation, presenting as pain, swelling, and progressive joint destruction.
Overview of the JAK-STAT pathway3,4,6,8,9
- A cytokine binds to a specific receptor on the cell surface...
- ...triggering JAK recruitment and activation inside the cell.
- The activated JAKs recruit and activate STATs...
- ...which in turn transmit the signal into the cell nucleus...
- ...initiating an inflammatory response that continues the cycle of pathologic inflammation.
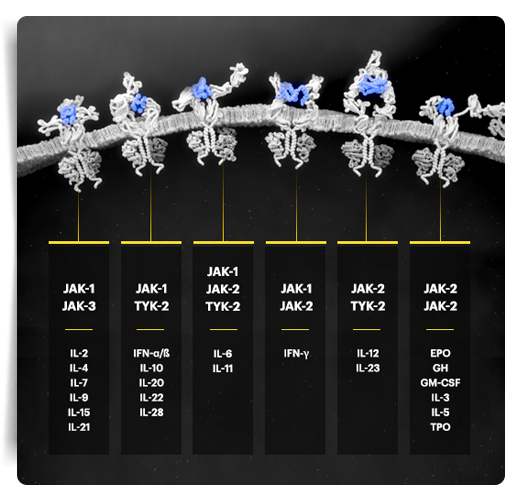
*The relevance of inhibition of specific JAK enzymes to therapeutic effectiveness is not currently known.1
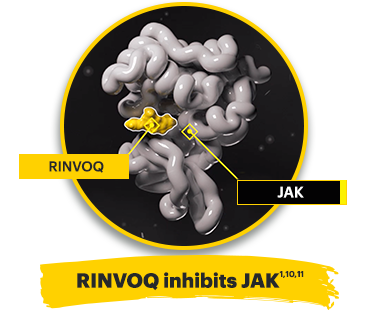
By inactivating JAK, RINVOQ is thought to block the recruitment, phosphorylation, and activation of STATs preventing the pro‑inflammatory cytokine signal from reaching the nucleus and inhibiting pro‑inflammatory processes associated with the JAK‑STAT pathway.
*The relevance of inhibition of specific JAK enzymes to therapeutic effectiveness is not currently known.1
Based on enzymatic and cellular assays, RINVOQ demonstrated greater inhibitory potency for JAK‑1 vs JAK‑2, JAK‑3, and TYK‑2.1,*
EPO=erythropoietin; GH=growth hormone; GM‑CSF=granulocyte‑macrophage colony‑stimulating factor; IFN=interferon; IL=interleukin; JAK=Janus kinase; PsA=psoriatic arthritis; STAT=signal transducer and activator of transcription; TPO=thrombopoietin; TYK=tyrosine kinase