A ONCE-DAILY ORAL JAK inhibitor indicated for the treatment of adults and pediatric patients 12+ years of age with refractory, moderate to severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies are inadvisable.1
WELL-STUDIED SAFETY PROFILE:
ADVERSE EVENTS
OF SPECIAL INTEREST2
Select baseline medical history in
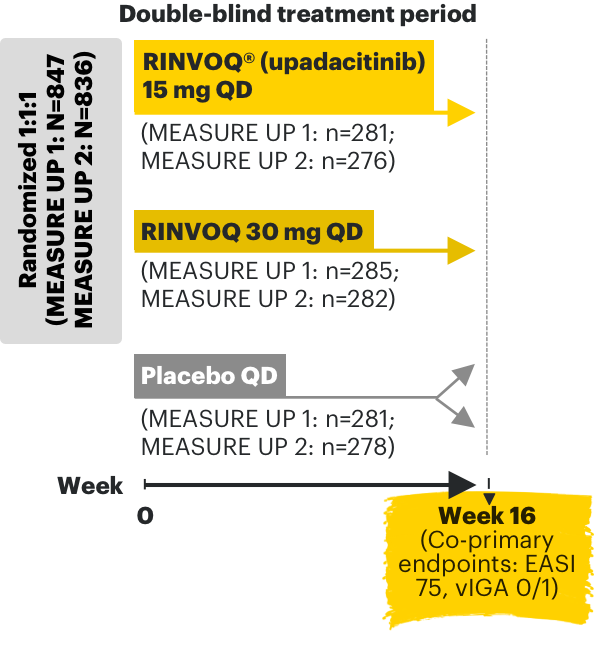
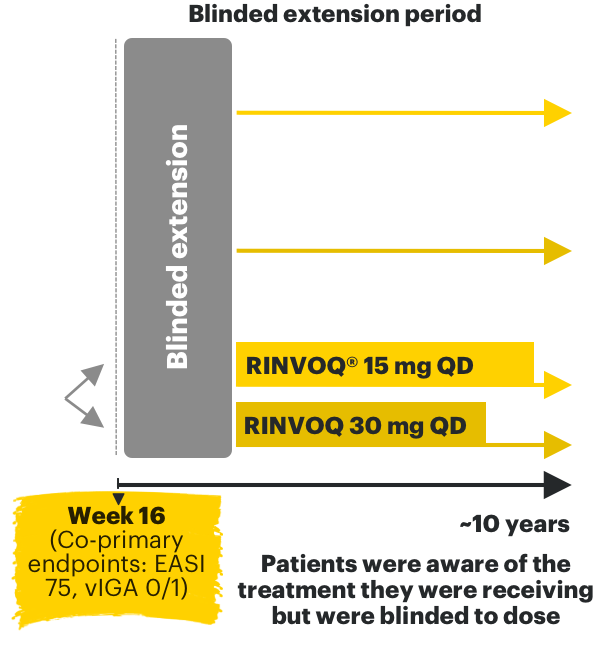
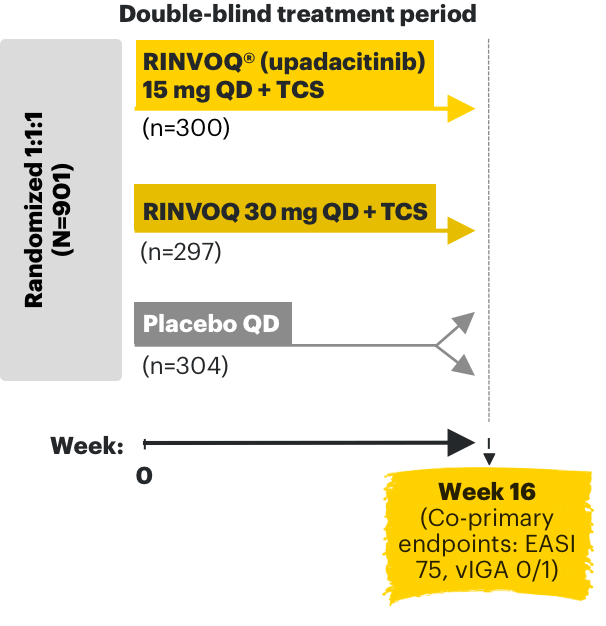
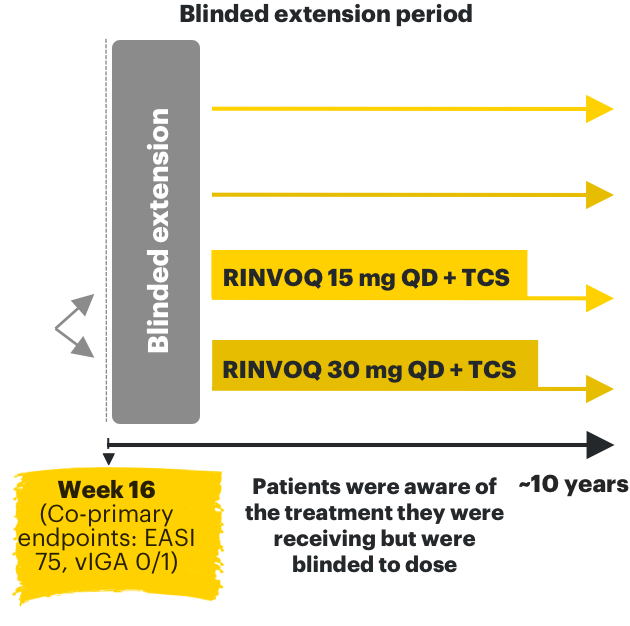
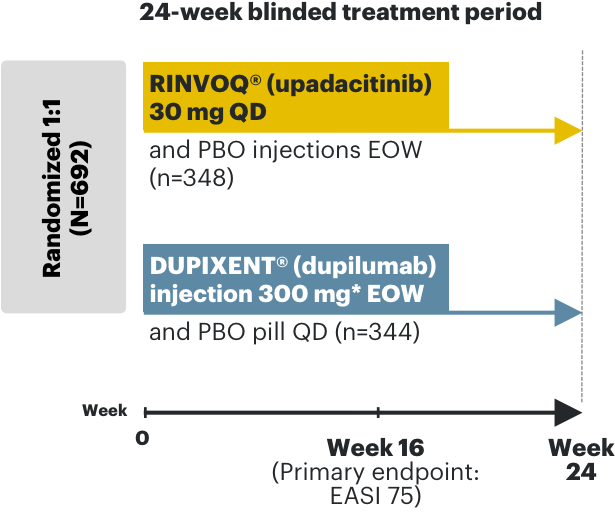
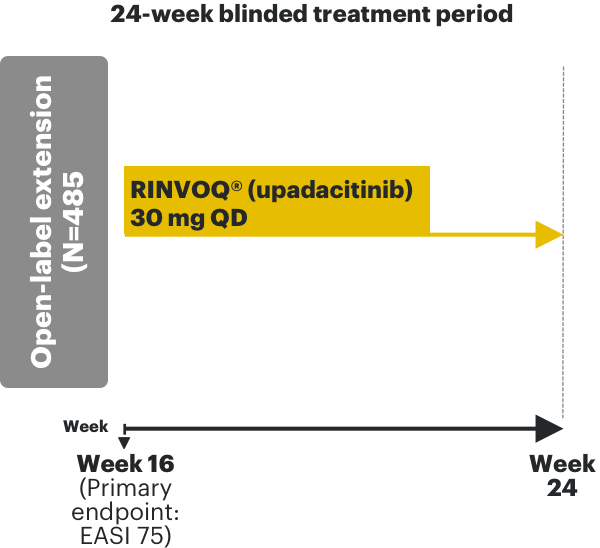
RINVOQ AD phase 3 clinical trials3
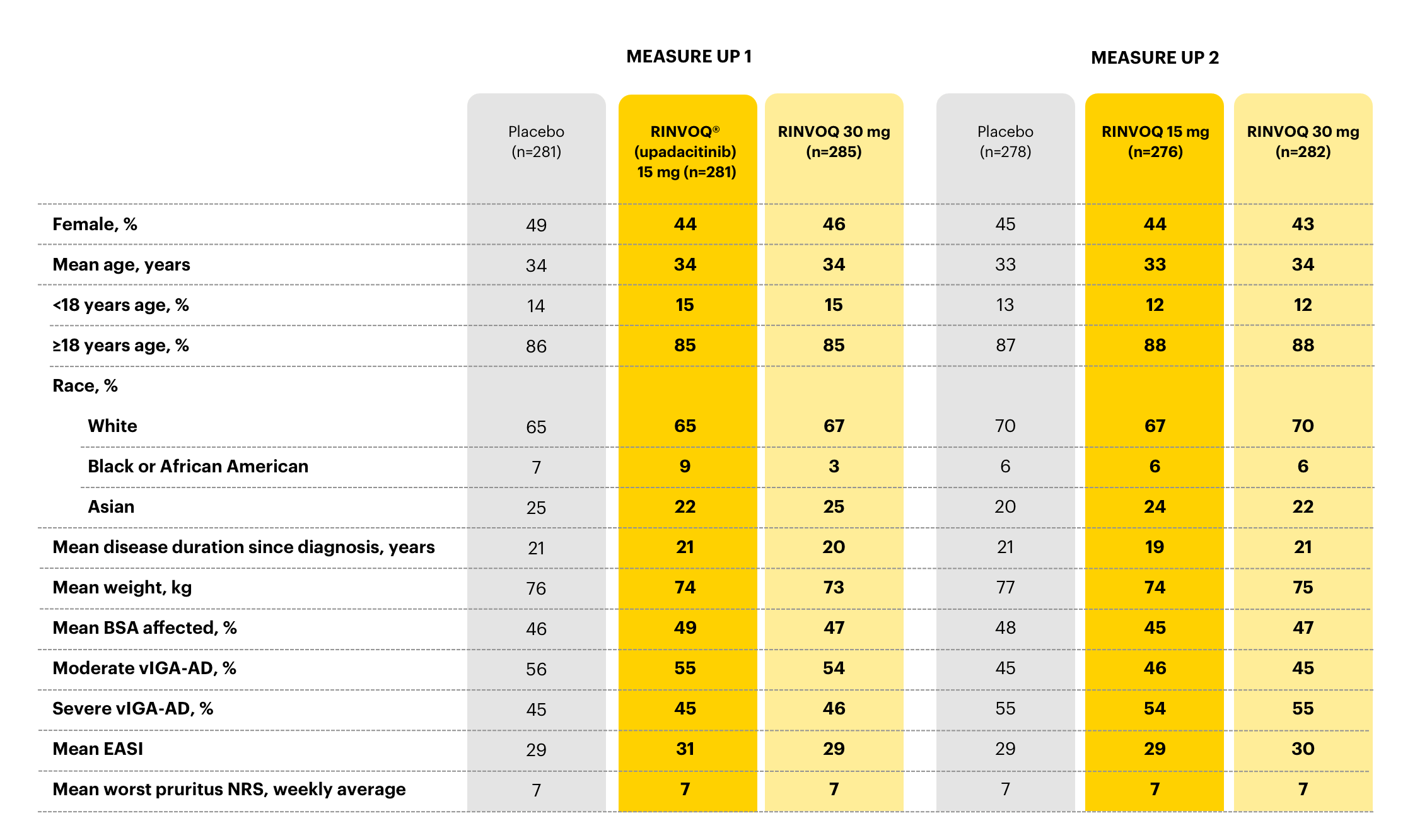
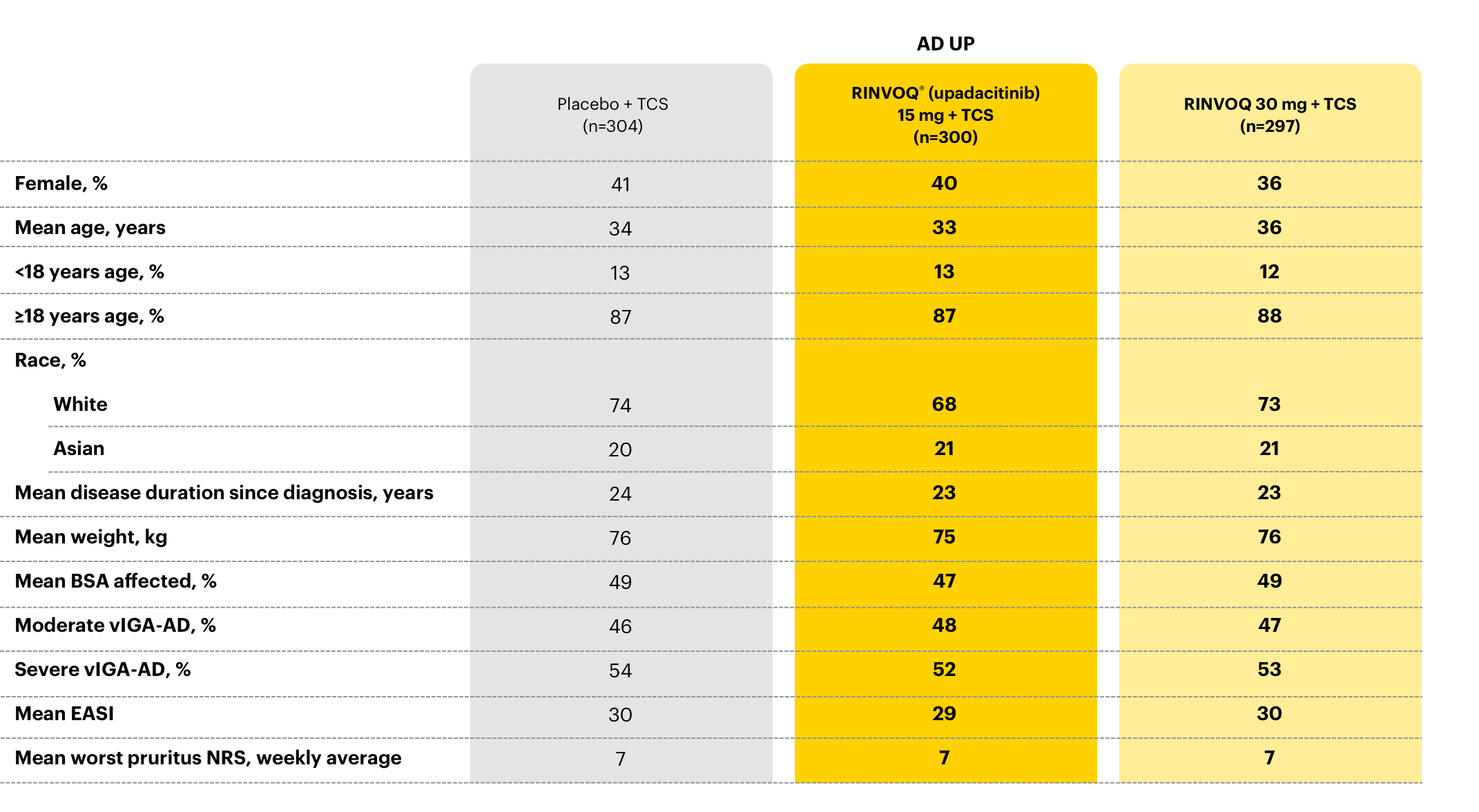
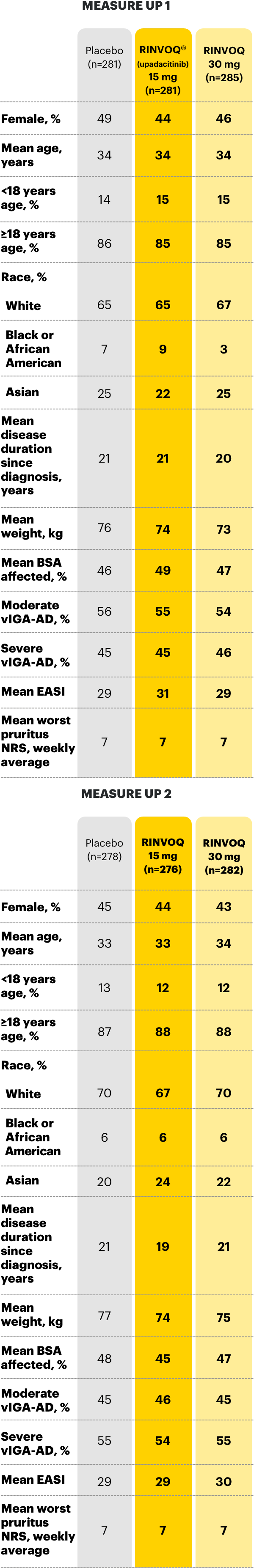
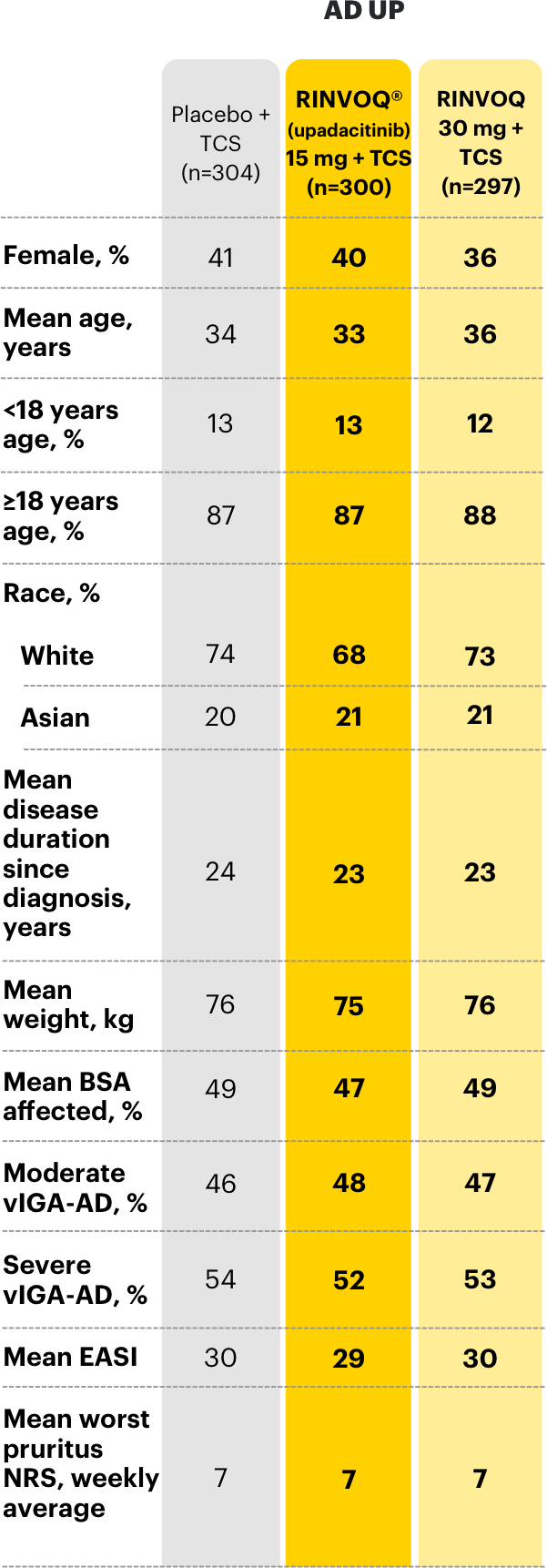
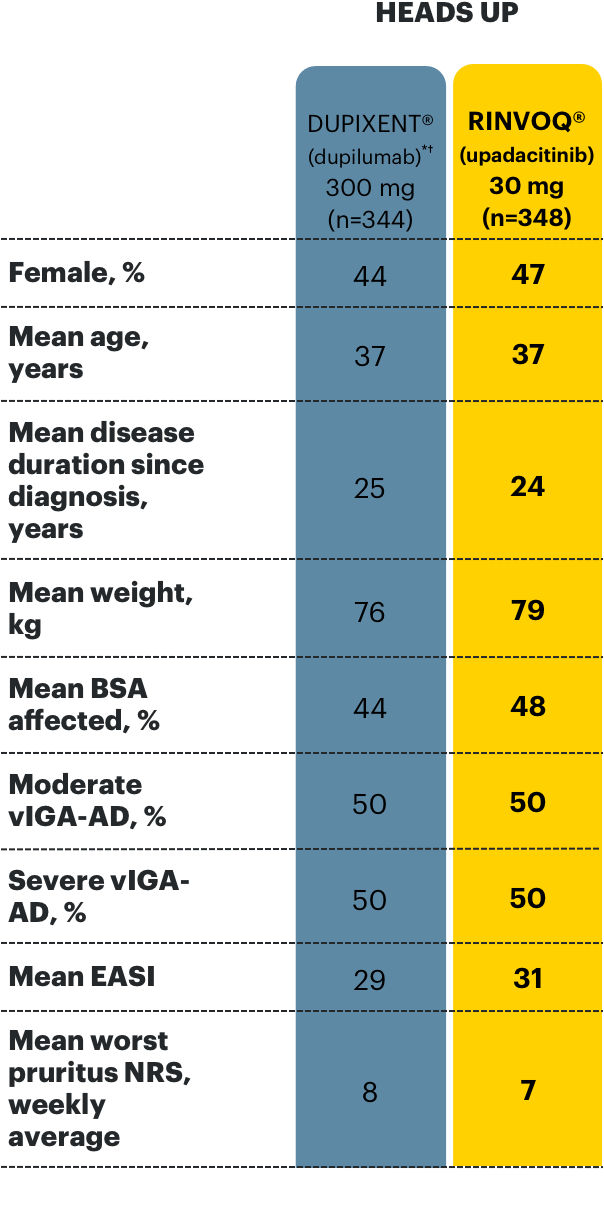
Select baseline characteristics in clinical trials3
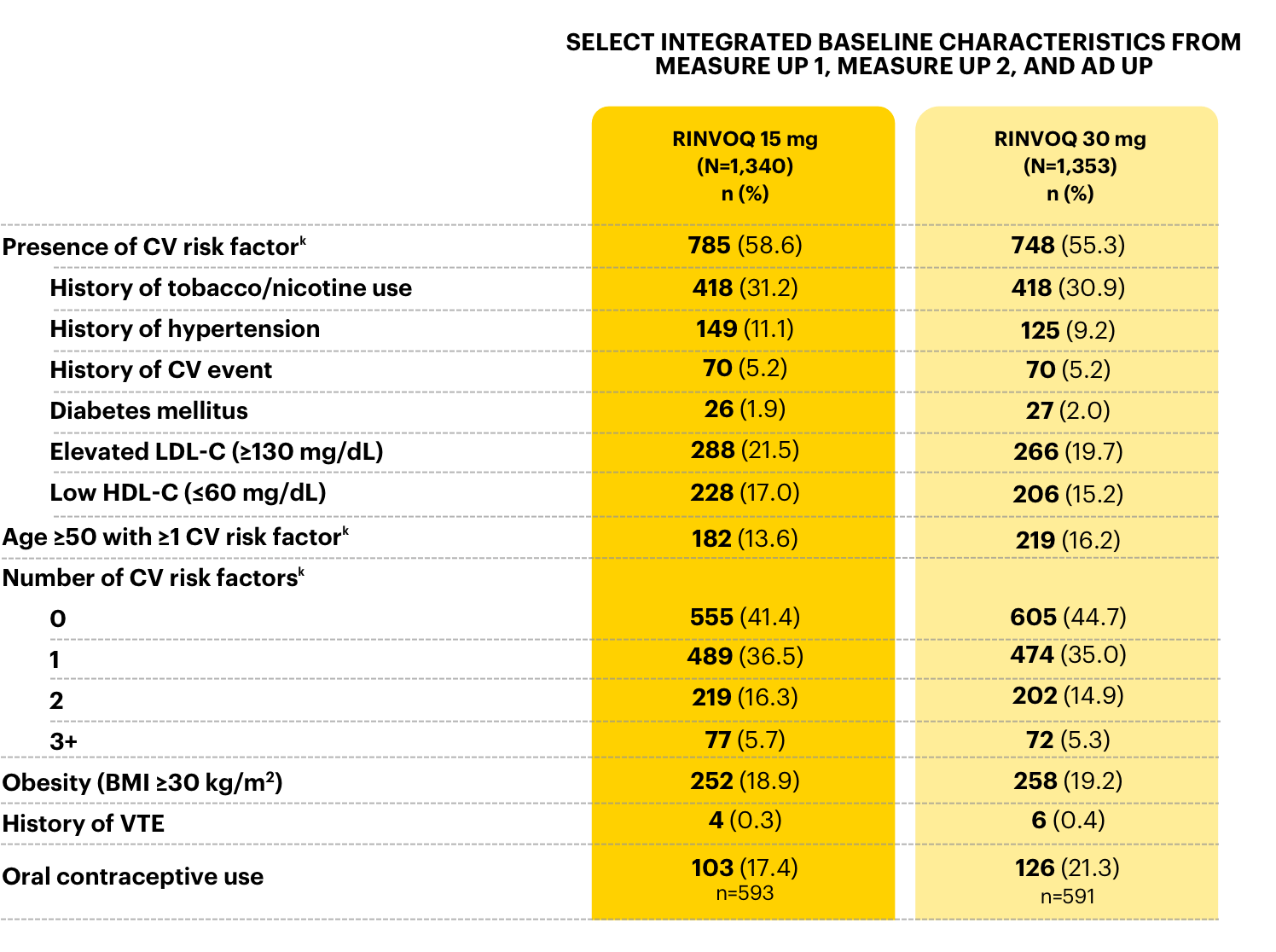
aCV risk factors included CV event, hypertension, diabetes mellitus, tobacco/nicotine use, elevated LDL-C, and lowered HDL-C.3
AD=atopic dermatitis; CHF=congestive heart failure; CV=cardiovascular; DVT=deep vein thrombosis; E/100 PY=events per 100 patient years; JAK=Janus kinase; LTE=long-term extension; n/100 PY=number of subjects with at least 1 event per 100 PY; NMSC=non-melanoma skin cancer; OCP=oral contraceptive pill; PE=pulmonary embolism; RA=rheumatoid arthritis; PY=patient years; TB=tuberculosis; TCS=topical corticosteroids; TNF=tumor necrosis factor; VTE=venous thromboembolic events; y/o=years old.
MACE: major adverse cardiovascular events, defined as cardiovascular death (includes acute myocardial infarction, sudden cardiac death, heart failure, cardiovascular procedure-related death, death due to cardiovascular hemorrhage, fatal stroke, pulmonary embolism and other cardiovascular causes), non-fatal myocardial infarction and non-fatal stroke.
RINVOQ WAS STUDIED IN
PATIENTS 12-75 YEARS OLD WITH VARIOUS COMORBIDITIES3
>50%
had at least 1 CV risk factora
>30%
were current or former smokers
~20%
of females were using OCP
~15%
were ≥50 years of age with ≥1 CV risk factora
Consider benefits and risks for individual patients prior to initiating or continuing therapy with RINVOQ, particularly in those who are current or past smokers and patients with other CV risk factors and those at increased risk for VTE. Avoid RINVOQ in patients at risk for thrombosis. Patients with symptoms of thrombosis should discontinue RINVOQ and be promptly evaluated. Discontinue RINVOQ in patients that have experienced myocardial infarction or stroke.
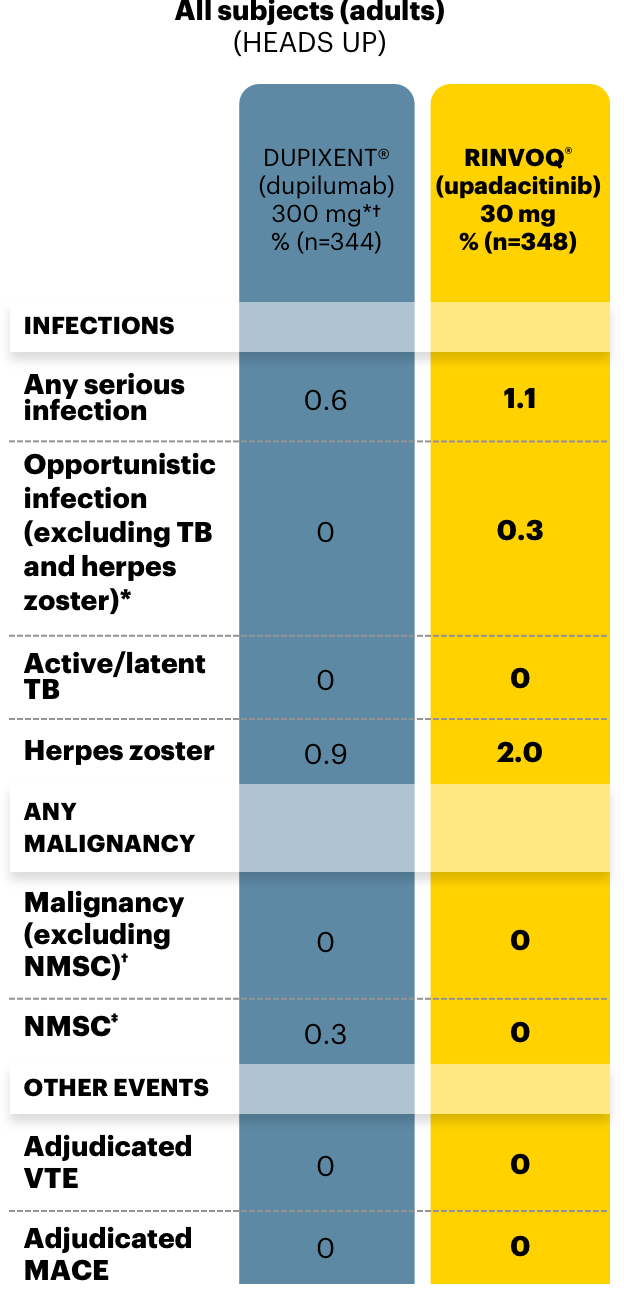
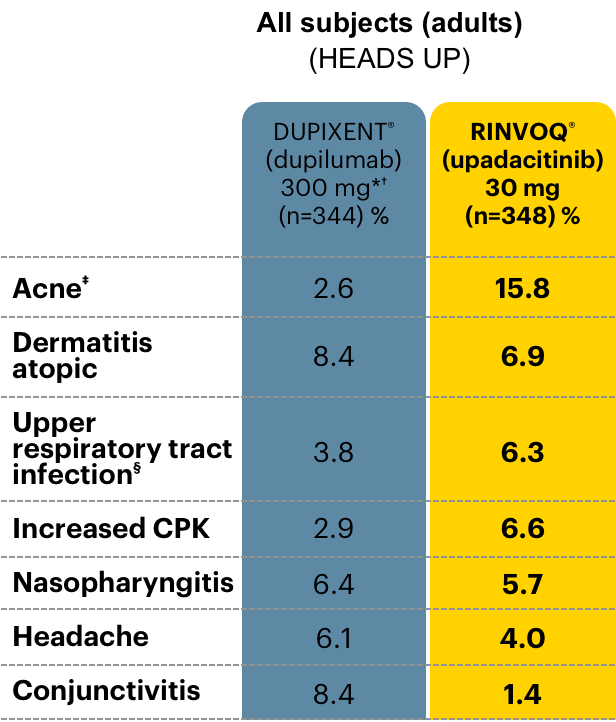
A closer look at adverse events (AEs) of special interest
Data as of June 30, 2021
Serious Infections
Adverse events of infection in all subjects: long-term integrated safety2
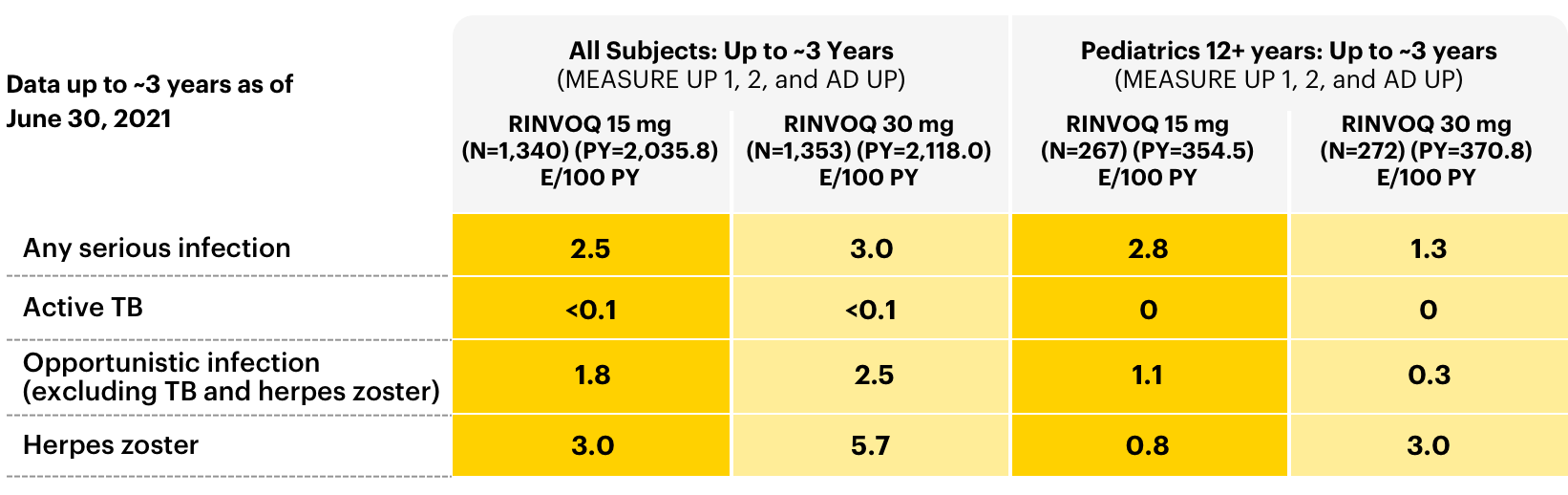
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
Safety Considerations1
Patients treated with RINVOQ are at increased risk for developing serious infections that may lead to hospitalization or death. These infections include tuberculosis (TB), invasive fungal, bacterial, viral, and other infections due to opportunistic pathogens. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids.
Mortality
Adverse events leading to death in all subjects: long-term integrated safety2
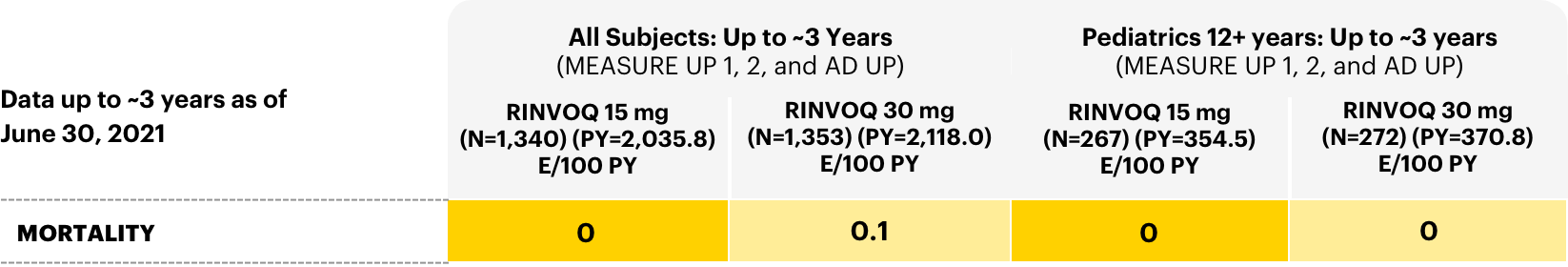
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
Safety Considerations1
A higher rate of all-cause mortality, including sudden cardiovascular (CV) death, was observed with a Janus kinase (JAK) inhibitor in a study comparing another JAK inhibitor with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients ≥50 years of age with at least one CV risk factor.
Malignancies
Adverse events of malignancy in all subjects: long-term integrated safety2
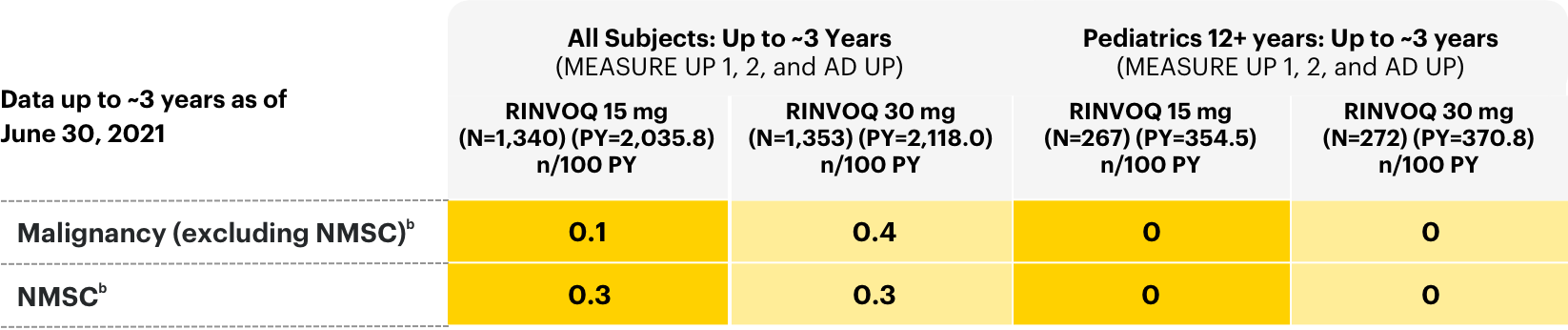
bRates shown are n/100 PY=number of subjects with at least 1 event per 100 PY. Per protocol, patients experiencing AEs of malignancy (except for localized NMSC or cancer of the cervix in situ) and adjudicated VTE were required to discontinue therapy.
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
Safety Considerations1
Lymphoma and other malignancies have been observed in RINVOQ-treated patients. A higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]), lymphomas, and lung cancer (in current or past smokers) was observed with another JAK inhibitor when compared with TNF blockers in RA patients. Patients who are current or past smokers are at additional increased risk.
NMSCs have been reported in patients treated with RINVOQ. Periodic skin examination is recommended for patients who are at increased risk for skin cancer. Advise patients to limit sunlight exposure by wearing protective clothing and using sunscreen.
Major Adverse Cardiovascular Events
Adverse events of adjudicated MACE in all subjects: long-term integrated safety2
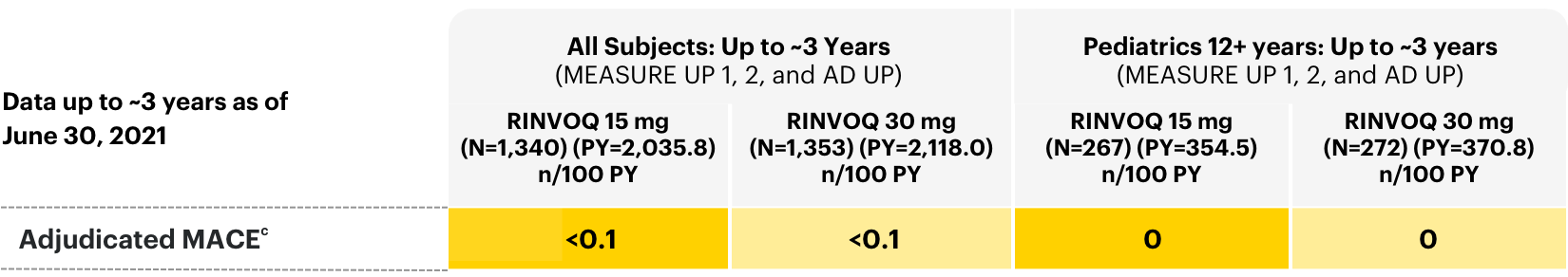
cRates shown are n/100 PY=number of subjects with at least 1 event per 100 PY. Per protocol, patients experiencing AEs of malignancy (except for localized NMSC or cancer of the cervix in situ) and adjudicated VTE were required to discontinue therapy.
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
Safety Considerations1
A higher rate of CV death, myocardial infarction, and stroke was observed with a JAK inhibitor in a study comparing another JAK inhibitor with TNF blockers in RA patients ≥50 years of age with at least one CV risk factor. Current or past smokers are at additional increased risk.
Thrombosis
Adverse events of adjudicated VTE in all subjects: long-term integrated safety2
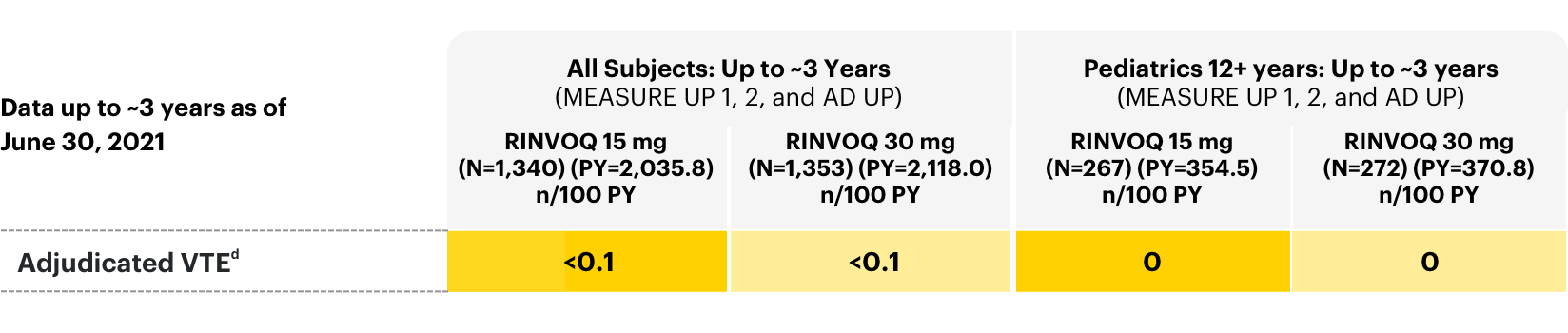
dRates shown are n/100 PY=number of subjects with at least 1 event per 100 PY. Per protocol, patients experiencing AEs of malignancy (except for localized NMSC or cancer of the cervix in situ) and adjudicated VTE were required to discontinue therapy.
Adverse reaction rates observed in clinical trials may not fully characterize the risks of RINVOQ. Certain adverse events may require longer observation periods and longer-term patient exposure to ascertain risk.
Safety Considerations1
Thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated with JAK inhibitors used to treat inflammatory conditions. A higher rate of thrombosis was observed with another JAK inhibitor when compared with TNF blockers in RA patients 50 years of age and older with at least one cardiovascular risk factor.