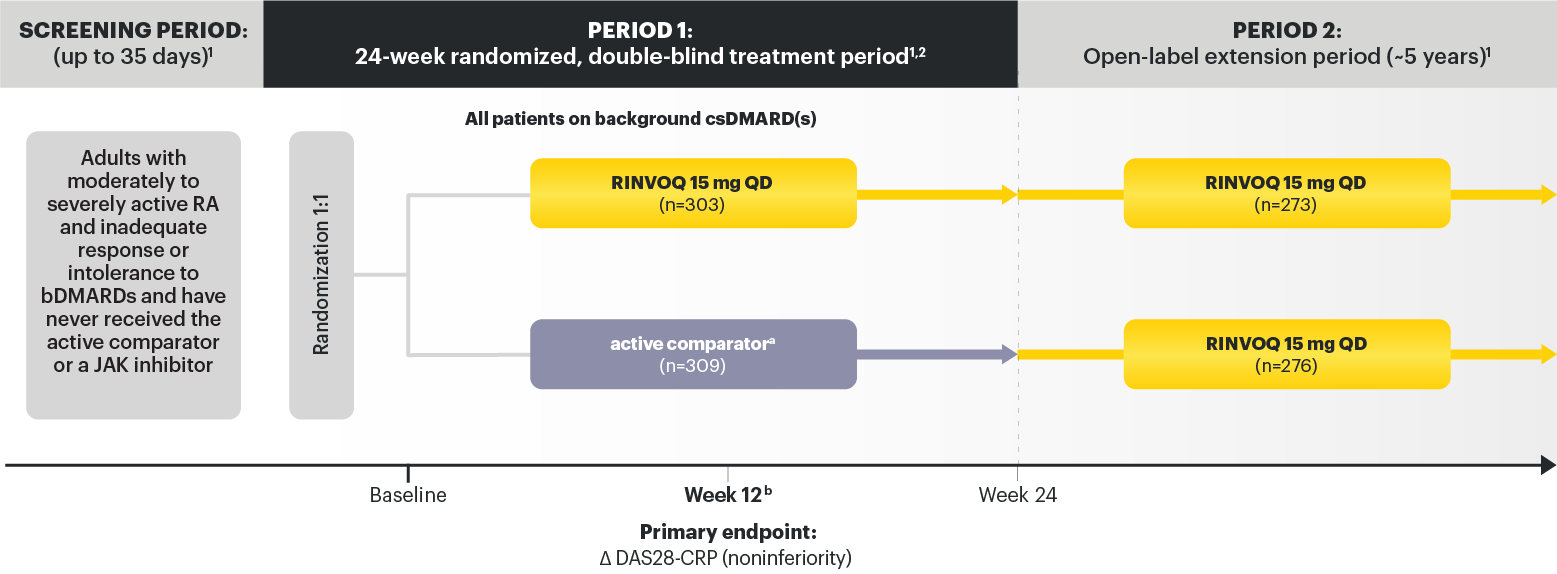
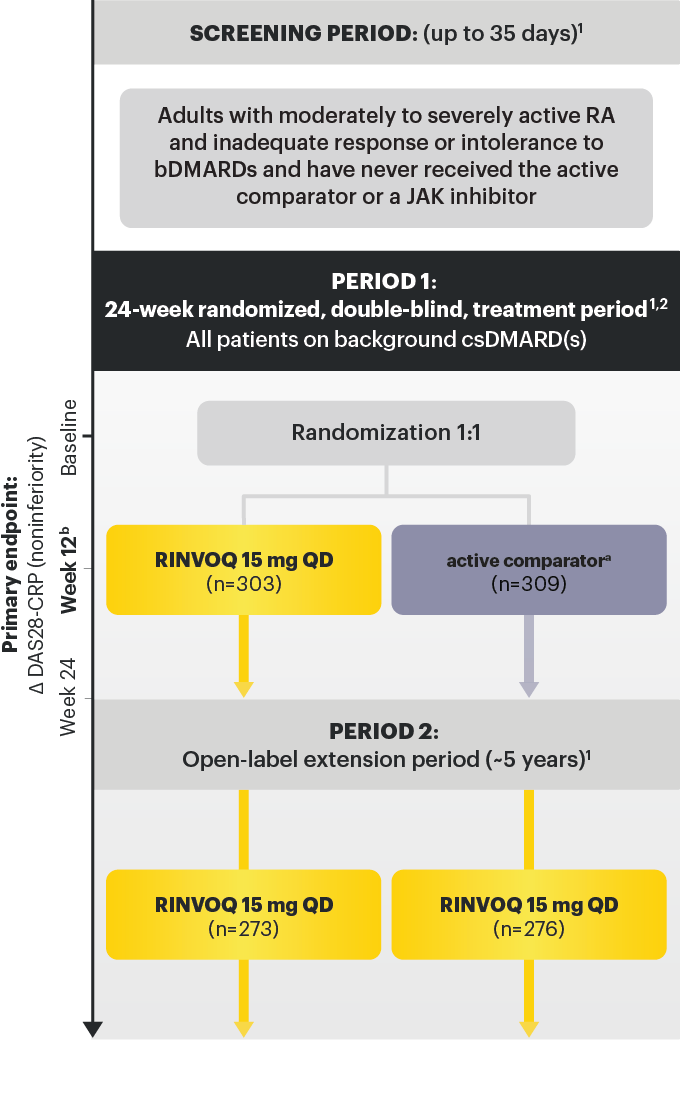
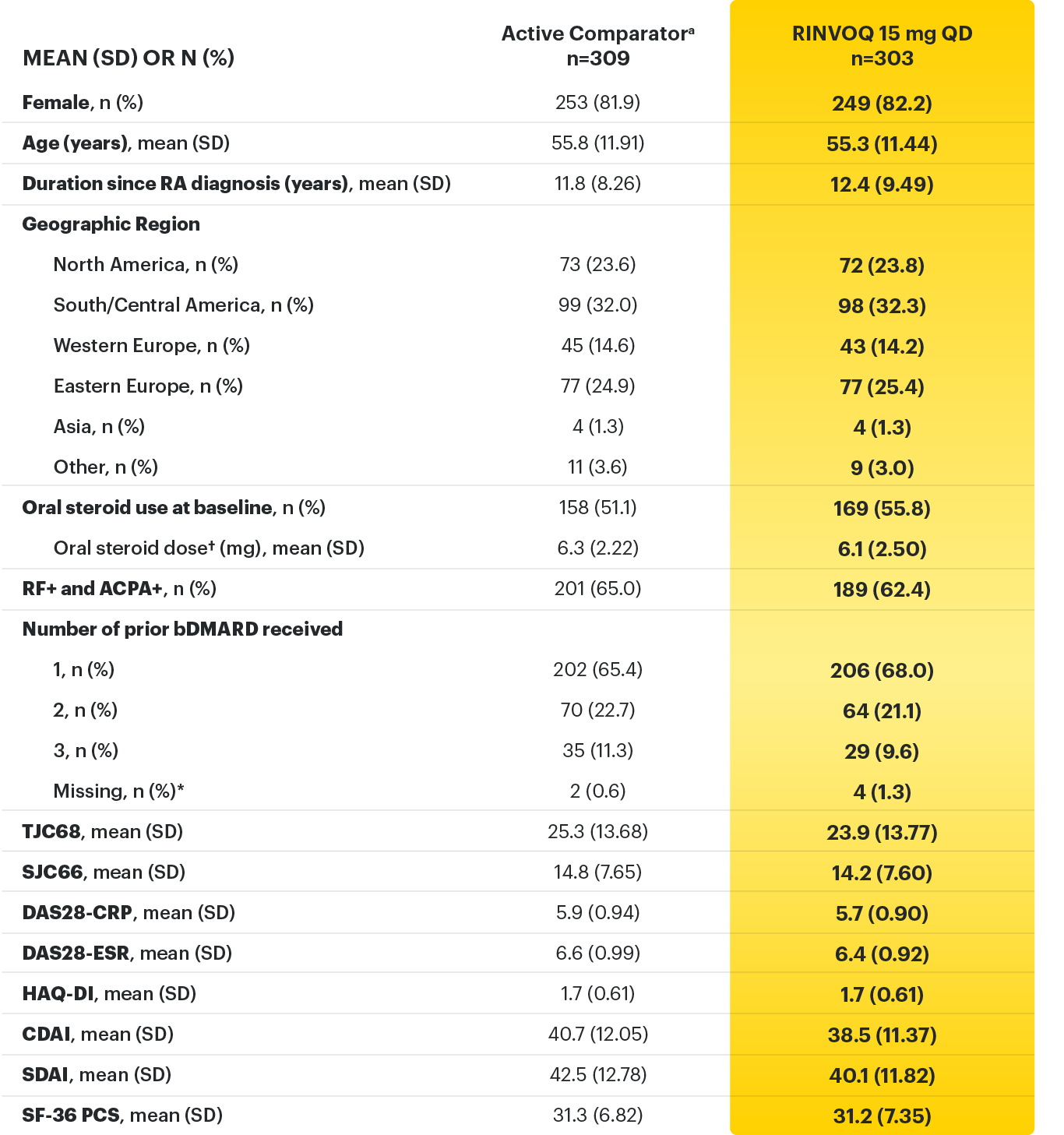
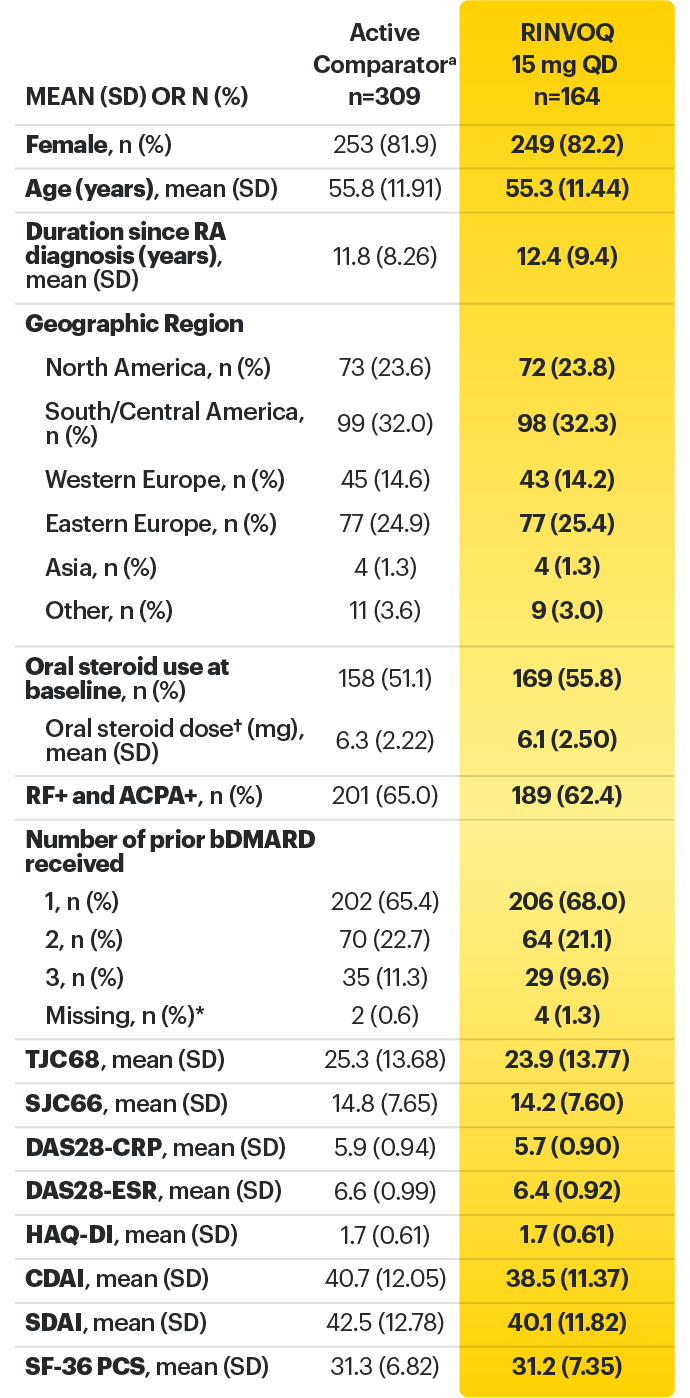
For moderate to severe rheumatoid arthritis (RA) in adult TNFi-IR patients1
Get started with the
Enrollment & Prescription Form
IR=intolerance or inadequate response; TNFi=tumor necrosis factor inhibitor
RINVOQ is for moderate to severe rheumatoid arthritis (RA) in adult TNFi-IR patients1
EXCEPTIONAL COMMERCIAL COVERAGE
FOR RINVOQ (upadacitinib) IN RA
PAYERS COVER RINVOQ
WITH >95%
>95%
PREFERRED COMMERCIAL AND MEDICARE PART D COVERAGE2,*,†
National Commercial and Medicare Part D formulary coverage under the pharmacy benefit as of January 20242
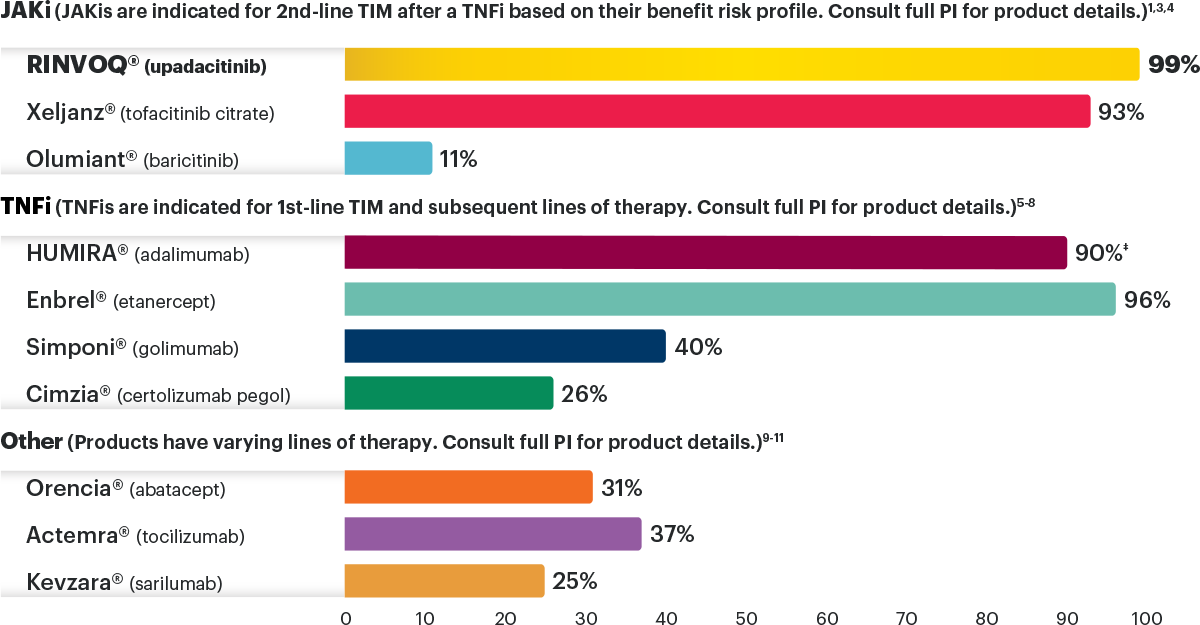
National commercial plan preferred formulary status for TIM therapies for moderate to severe RA under the pharmacy benefit as of January 20242,*,†,‡
Preferred coverage also could mean a STANDARD PRIOR AUTHORIZATION (PA) and APPEALS PROCESS, potential for one-time PA/appeal approval.
*RINVOQ is on a preferred tier or otherwise has preferred status on the plan’s formulary.
†Coverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
‡Percentages reflect anticipated coverage.
JAKi=Janus kinase inhibitor; PI=prescribing information; RA=rheumatoid arthritis; TIM=targeted immunomodulator; TNFi=tumor necrosis factor inhibitor
Preferred coverage also could mean a
STANDARD PRIOR AUTHORIZATION (PA)
and APPEALS PROCESS, potential for one-time PA/appeal approval.
Cimzia, Actemra, and Orencia are available as products on medical benefits plans.
Most national and regional health plans may not approve non-preferred products for patients who have shown clinical stability solely through the use of samples or other free goods.
Products listed here are not interchangeable with one another. Not all products are shown, including biosimilars. Material differences exist between the listed products, including with respect to safety profiles and line of therapy. Consult each individual product's USPI for full details.
No conclusions regarding comparative safety or efficacy can be drawn from this information. Selection of a treatment regimen should be individualized for each patient based on factors including, but not limited to, product efficacy, product safety profile, adverse events, dosage and administration, potential for drug interactions, patients’ test results, and comorbid conditions.

*RINVOQ is on a preferred tier or otherwise has preferred status on the plan’s formulary.
†Coverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
‡Percentages reflect anticipated coverage.
JAKi=Janus kinase inhibitor; PI=prescribing information; RA=rheumatoid arthritis; TIM=targeted immunomodulator; TNFi=tumor necrosis factor inhibitor
Please see HUMIRA full Prescribing Information.
Look up commercial insurance plans
for RINVOQ in your area
- Plan Name
- Plan Type
EXCEPTIONAL MEDICARE
PART D COVERAGE FOR RINVOQ (upadacitinib) in RA
PAYERS COVER RINVOQ
>95%
>95%
PREFERRED COMMERCIAL AND MEDICARE PART D COVERAGE2,*,†
National Commercial and Medicare Part D formulary coverage under the pharmacy benefit as of January 20242
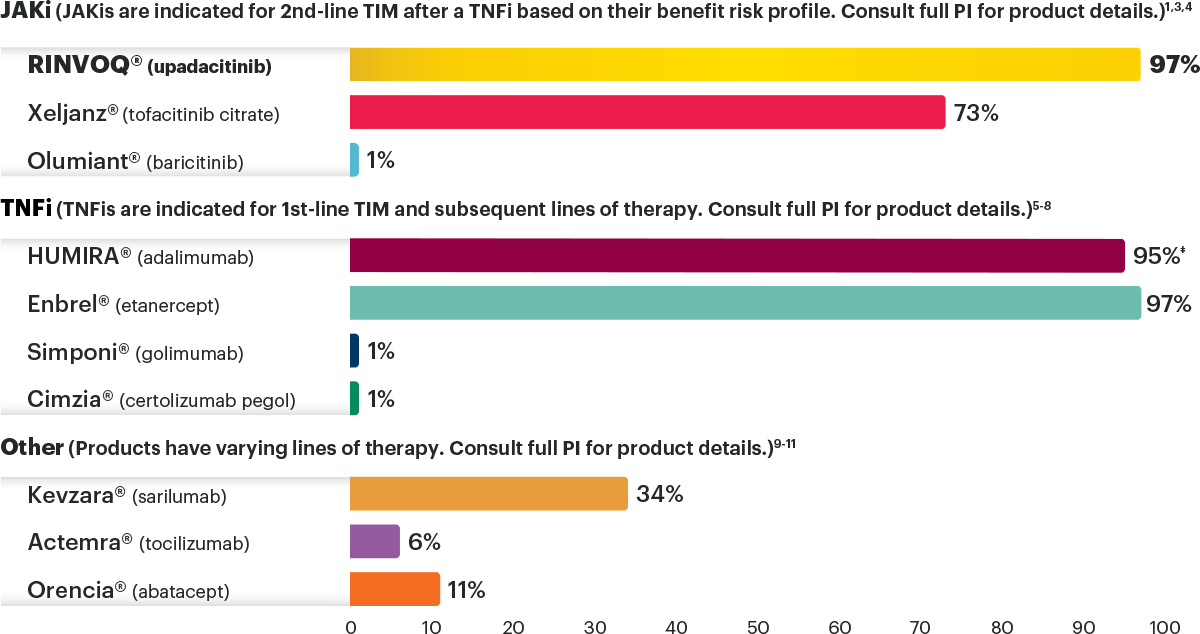
Medicare Part D plan preferred formulary status for TIM therapies for moderate to severe RA under the pharmacy benefit as of January 2024 2,*,†,‡
*RINVOQ is on a preferred tier or otherwise has preferred status on the plan's formulary.
†Coverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
‡Percentages reflect anticipated coverage.
JAKi=Janus kinase inhibitor; PI=prescribing information; RA=rheumatoid arthritis; TIM=targeted immunomodulator; TNFi=tumor necrosis factor inhibitor
Cimzia, Actemra, and Orencia are available as products on medical benefits plans.
Most national and regional health plans may not approve non-preferred products for patients who have shown clinical stability solely through the use of samples or other free goods.
Products listed here are not interchangeable with one another. Not all products are shown, including biosimilars. Material differences exist between the listed products, including with respect to safety profiles and line of therapy. Consult each individual product's USPI for full details.
No conclusions regarding comparative safety or efficacy can be drawn from this information. Selection of a treatment regimen should be individualized for each patient based on factors including, but not limited to, product efficacy, adverse events, dosage and administration, potential for drug interactions, patients’ test results, and comorbid conditions.
*RINVOQ is on a preferred tier or otherwise has preferred status on the plan’s formulary.
†Coverage requirements and benefit designs vary by payer and may change over time. Please consult with payers directly for the most current reimbursement policies.
‡Percentages reflect anticipated coverage.
JAKi=Janus kinase inhibitor; PI=prescribing information; RA=rheumatoid arthritis; TIM=targeted immunomodulator; TNFi=tumor necrosis factor inhibitor
Please see HUMIRA full Prescribing Information.
Two products.
One exceptional patient experience.



NURSE
AMBASSADORS*
Empower patients
Our Nurse Ambassadors are the heart of RINVOQ Complete and HUMIRA Complete
Nurse Ambassadors provide 1:1 support to help patients start and stay on track with their prescribed treatment plan, including:
- Help patients understand the importance of following the treatment plan prescribed by their healthcare professional.
- Committed to answering questions throughout the experience to help limit treatment disruptions.
- Answer patients’ insurance questions and connect them with additional insurance expertise.
- Identify ways for patients to save on prescription costs.

ACCESS
SPECIALISTS
Insurance support when needed
- Resource with expertise on Medicare and commercial plans at a national, local, and program level so that they can educate on potential options to consider based on each patient’s unique financial situation.
- Can educate on payer prior authorization and appeal processes so you can determine the best access option for each patient’s unique situation.

ACCESS & SAVINGS
Help with access &
treatment affordability
Complete can help your commercial patients save:
- With the Complete Savings Card, your eligible commercially insured patients may pay as little as $5 per month.†
- Complete may help eligible commercially insured RINVOQ patients experiencing initial coverage delays or denials access their prescribed therapy at no charge while coverage is established.‡
Have questions or need support over the phone?
Call 1-877-COMPLETE (1‑800‑274‑6867)
*Nurse Ambassadors are provided by AbbVie and do not provide medical advice or work under the direction of the prescribing healthcare professional (HCP). They are trained to direct patients to speak with their HCP about any treatment‑related questions, including further referrals.
†Eligibility: Available to patients with commercial insurance coverage for RINVOQ® (upadacitinib), or HUMIRA® (adalimumab) who meet eligibility criteria. This co-pay assistance program is not available to patients receiving prescription reimbursement under any federal, state, or government-funded insurance programs (for example, Medicare [including Part D], Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs) or where prohibited by law. Offer subject to change or termination without notice. Restrictions, including monthly maximums, may apply. This is not health insurance. For full Terms and Conditions, visit RINVOQSavingsCard.com for RINVOQ® patients, and HUMIRASavingsCard.com for HUMIRA® patients, or call 1.800.2RINVOQ or 1.800.4HUMIRA for additional information To learn about AbbVie’s privacy practices and your privacy choices, visit https://abbv.ie/corpprivacy
‡Eligibility criteria: Available to patients aged 63 or younger with commercial insurance coverage. Patients must have a valid prescription for RINVOQ® (upadacitinib) for an FDA approved indication and a denial of insurance coverage based on a prior authorization request on file along with a confirmation of appeal. Continued eligibility for the program requires the submission of an appeal of the coverage denial every 180 days. Program provides for RINVOQ® (upadacitinib) at no charge to patients for up to two years or until they receive insurance coverage approval, whichever occurs earlier, and is not contingent on purchase requirements of any kind. Program is not available to patients whose medications are reimbursed in whole or in part by Medicare, Medicaid, TRICARE, or any other federal or state program. Offer subject to change or discontinuance without notice. This is not health insurance and program does not guarantee insurance coverage. No claims for payment may be submitted to any third party for product dispensed by program. Limitations may apply.
How to enroll patients in
RINVOQ complete
Help patients get the support they need to start and stay
on track with their prescribed treatment
To enroll a patient in RINVOQ Complete:

Pull your patient’s patient demographic sheet from your Electronic Health Record
- Ensure the following information is included: full home address, email address, medical and prescription insurance information, and any relevant clinical details.
- Redact the patient’s entire Social Security Number (if present).
- Failure to include the patient demographic sheet may result in delayed enrollment.

Download the enrollment & prescription form for your specialty

Fill out the form with your patient
- This form enrolls your patient and can be used to initiate a prescription with your patient's preferred specialty pharmacy.
- The RINVOQ Complete Prescription section may help your commercially insured patients get access to RINVOQ if they experience a delay or denial in their insurance coverage.*

Fax the enrollment & prescription form and the patient demographic sheet to 1-678-727-0690
- You will receive a call from an Access Specialist to discuss next steps.
- If using the Pharmacy Prescription section, fax a copy to your patient's specialty pharmacy as well.

Inform your patient that they have been enrolled
- Let your patient know to expect a call from their Ambassador.
*Eligibility criteria: Available to patients aged 63 or younger with commercial insurance coverage. Patients must have a valid prescription for RINVOQ® (upadacitinib) for an FDA approved indication and a denial of insurance coverage based on a prior authorization request on file along with a confirmation of appeal. Continued eligibility for the program requires the submission of an appeal of the coverage denial every 180 days. Program provides for RINVOQ® (upadacitinib) at no charge to patients for up to two years or until they receive insurance coverage approval, whichever occurs earlier, and is not contingent on purchase requirements of any kind. Program is not available to patients whose medications are reimbursed in whole or in part by Medicare, Medicaid, TRICARE, or any other federal or state program. Offer subject to change or discontinuance without notice. This is not health insurance and program does not guarantee insurance coverage. No claims for payment may be submitted to any third party for product dispensed by program. Limitations may apply.

COMPLETE APP
Track treatment
The Complete App is designed to help patients stay on track with their prescribed RINVOQ or HUMIRA treatment by helping them:
- Access additional resources, including a savings card for those that are eligible
- Set customized medication reminders
- Log and share symptoms with HCPs
- Log medication lot number and medication expiration date

COMPLETEPRO.COM
Streamline the Rx process
CompletePro.com enables seamless enrollment into RINVOQ Complete and HUMIRA Complete, and helps streamline the prescription process for your patients.
With CompletePro.com, you can:
- Request benefits verifications
- Complete and submit prior authorizations
- Send prescriptions to the patient’s specialty pharmacy of choice, with the option to include a savings card
- Receive alerts for annual reauthorizations and renewals
- Track and monitor where patients are in the prescription process
Learn more about streamlining the prescription process with Complete Pro:
Register now at COMPLETEPRO.COM
Download RINVOQ
resources to support
your
patients and your practice.
RINVOQ SAFETY DATA
Review the well-studied safety profile of RINVOQ,
including both short- and long-term analyses